This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To investigate the feasibility of ultrasound (US)-guided steroid injection by in-plane approach for cubital tunnel syndrome (CuTS), based on symptomatic, morphologic and electrophysiological outcomes.
Methods
A total of 10 patients, who were clinically diagnosed as CuTS and confirmed by an electrodiagnostic study, participated in this study. US-guided injection into the cubital tunnel was performed with 40 mg triamcinolone and 2 mL of 1% lidocaine. Outcomes of the injections were evaluated at pre-injection, 1st week and 4th week after injection. Visual analog scale, self-administered questionnaire of the ulnar neuropathy at the elbow (SQUNE), and McGowan classification were used for clinical evaluation. Cross-sectional area of the ulnar nerve by US and the electrophysiological severity scale through a nerve conduction study were utilized in the evaluation of morphologic and electrophysiological changes. The cross-sectional area of the ulnar nerve was measured at 3 points of condylar, proximal, and distal level of the cubital tunnel.
Results
No side effects were reported during the study period. The visual analog scale and cross-sectional area showed a significant decrease at 1st week and 4th week, as compared to baseline (p<0.05). The electrophysiological severity scale was significantly decreased at the 4th week, as compared with baseline and 1st week (p<0.05). Among the quantitative components of the scale, there were statistically significant improvements with respect to the conduction velocity and block.
Conclusion
The new approach of US-guided injection may be a safe tool for the treatment of CuTS. Symptomatic and morphologic recoveries preceded the electrophysiological improvement.
Go to :

Keywords: Cubital tunnel syndrome, Ulnar nerve, Neural conduction, Ultrasonography, Injections
INTRODUCTION
Cubital tunnel syndrome (CuTS) ranks second after carpal tunnel syndrome, as a common cause of entrapment neuropathy in the upper extremity with an incidence of 24.7 per 100,000 people [
1,
2]. Ulnar nerve compression of the cubital tunnel during repeated elbow flexion can cause frequent friction and increased pressure on the ulnar nerve, which may lead to ulnar neuropathy presenting as paresthesia. This may involve the hypothenar region, pain at the elbow, sensory symptoms with prolonged flexion of the elbow, and in severe cases motor deficit of the ulnar nerve innervated hand muscles [
3].
The ulnar neuropathy at the elbow may be treated by surgical intervention or conservative therapy including corticosteroid injection [
2]. Despite the high incidence and disease severity associated with CuTS, there are few reports regarding steroid injection therapy specifically as a non-surgical treatment, in contrast to a number of studies related to carpal tunnel syndrome [
4,
5]. However, a few studies have examined ultrasound (US)-guided injection as a treatment option for CuTS, but failed to show consensus in methodological guidance [
6,
7]. Recently, a cadaveric study demonstrated real-time visualized US-guided injection by in-plane approach for cubital tunnel that can accurately provide the injection. This approach can also identify dynamic morphologic changes and size differences in the cubital tunnel after injection [
8]. The cadaveric study findings suggested that this approach can be potentially translated to clinical applications and improve the outcome in live patients with CuTS.
Therefore, in this study, we applied this new in-plane technique of the real-time visualized US guidance of steroid injection therapy for patients with CuTS. This study aimed to verify the clinically relevant therapeutic effects on symptomatic, morphologic, and electrophysiological responses of the ulnar nerve.
Go to :

MATERIALS AND METHODS
Subjects
Ten patients who were clinically diagnosed as CuTS and confirmed by an electrodiagnostic study were recruited to participate in the study. All patients presented with symptoms of CuTS, such as pain, numbness, or sensory changes along the ulnar path. If patients complained of bilateral symptoms, the side exhibiting more severe symptoms was studied. Patients were excluded when they reported any systemic diseases associated with polyneuropathy, cervical radiculopathy, history of trauma to the elbow lesion or previous surgical repair for the ulnar nerve. The research protocol was approved by the local medical ethics committee, and all patients provided informed consent.
Methods
All patients were injected under US guidance for the CuTS. Before injection, patients were examined for the Tinel sign by tapping over the medial elbow, as well as Wartenberg and Froment signs that are indicative of weakness in the distal ulnar-innervated muscles, third palmar interosseous and adductor pollicis, respectively. Patients were assessed with respect to the symptomatic, morphologic, and electrophysiological changes, at the times of pre-injection, and the 1st and 4th week after-injection.
The degree of the symptoms was assessed with objectively measurable scores including visual analog scale, McGowan score, and self-administered questionnaire of the ulnar neuropathy at the elbow (SQUNE). McGowan score is a grading system for the severity of ulnar neuropathy with grades 1 to 3 (mild to severe) [
9]. SQUNE includes 9 questions regarding patients' symptoms, and patients are asked to score each question from 1 (absence of symptom) to 5 (most severe) [
10].
Each patient also underwent an electrodiagnostic study to confirm the diagnosis of CuTS and to assess the electrophysiological severity scale for the ulnar nerve. The electrodiagnostic study consisted of a motor and antidromic sensory nerve conduction test, as well as needle electromyography. These tests were performed by a single physiatrist using a Keypoint EMG system (Dantec, Copenhagen, Denmark). The American Association of Neuromuscular & Electrodiagnostic Medicine guidelines were used to diagnose patients with CuTS [
11] and are as follows: 1) absolute slowing (<50 m/s) of the motor conduction velocity across the elbow and 2) motor conduction velocity across the elbow at least 10 m/s less than the motor conduction velocity in the forearm segment from the elbow to the wrist [
12].
An ordinal scale was used, and the total scale was measured as the sum of scores from each domain to assess the electrophysiological severity scale. The four domains were the degree of damage for the motor conduction velocity, the amplitude of compound muscle action potential (CMAP), sensory conduction velocity and amplitude, and electromyography of distal ulnar-innervated muscles [
13]. Furthermore, across the elbow, the difference in motor conduction velocity and drop rate of CMAP amplitude was calculated, for the evaluation of velocity decrease and conduction block, respectively. Drop rate of CMAP amplitude is calculated by subtracting the below elbow's CMAP amplitude from the above elbow's CMAP amplitude and dividing by below elbow's CMAP amplitude, multiplied by 100 for the percentage of neural damage. In CuTS, a drop in amplitude in excess of 20% is occasionally encountered when stimulating above, as compared to below the elbow [
11].
US was used to guide the injection procedure and measure the size of ulnar nerve for evaluation of morphologic changes with an Accuvix V20 machine (Medison, Seoul, Korea) with a 7-12 MHz linear array transducer. During the evaluation, the patients were placed in a supine position with the shoulder abducted and the elbow flexed at 90°. The cross-sectional area of the ulnar nerve was measured at the level of the epicondyle, 2 cm proximal and distal to the epicondyle.
All patients were injected with a combination of 2 mL of 1% lidocaine and 40 mg of triamcinolone acetonide under US guidance with in-plane technique that was recently studied in cadaveric specimens [
8]. Firstly, the ulnar nerve within the cubital tunnel was scanned and identified in the transverse plane. Secondly, a 25-guage needle was inserted into the cubital tunnel between the medial epicondyle and the ulnar nerve at the level of the epicondyle, advancing from the medial side to the olecranon. After injection, we confirmed that the ulnar nerve was separated from the epicondyle due to hydrodissection (
Fig. 1).
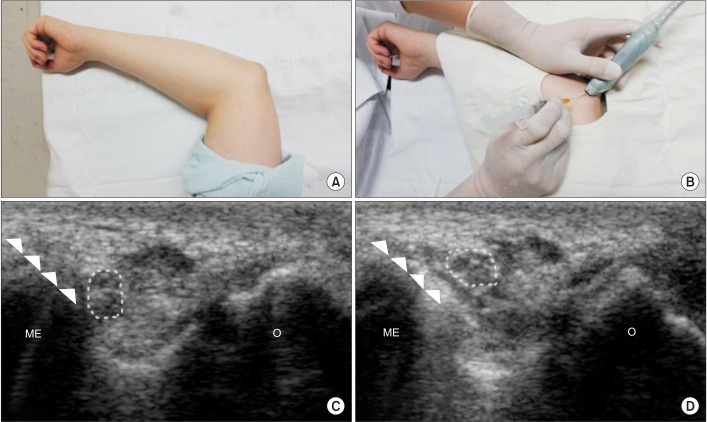 | Fig. 1Ultrasound-guided injection into the cubital tunnel was conducted via an in-plane technique. (A) The patient was placed in a supine position with the shoulder abducted and the elbow flexed at 90°. (B) The ulnar nerve within the cubital tunnel was identified in transverse plane, and the injection was conducted after aseptic preparation. (C) The needle (arrowhead) passed between the medial epicondyle (ME) and ulnar nerve (dotted circle) at the level of the epicondyle. (D) After the injection, we confirmed that the ulnar nerve was separated from the epicondyle by the effect of hydrodissection. O, olecranon.
|
Statistics
Comparisons of before and after treatment measurements were performed using Wilcoxon singed-rank test. Analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) and p-values <0.05 were considered significant.
Go to :

RESULTS
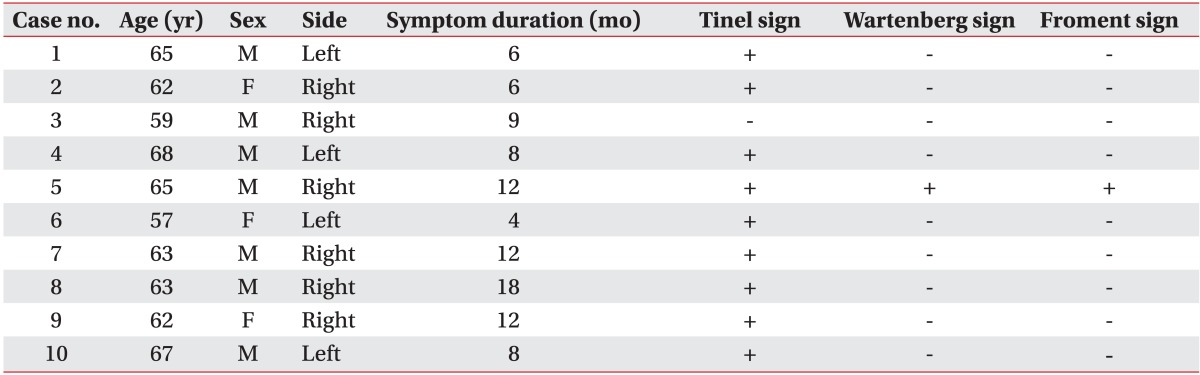
After injecting 10 patients who were diagnosed with CuTS, the patients were evaluated at the time of pre-injection, and at 1st week and 4th week after injection. The mean age of patients was 63.1 years and the mean duration of symptoms was 9.5 months at the baseline of pre-injection. A positive Tinel sign was found in 9 patients, however, positive Wartenberg and Froment signs were found in only 1 patient (
Table 1). There were no procedural side effects immediately after the injection and during the follow-up period.
Table 1
General characteristics and clinical signs of the patients (n=10)

Among the symptom scores, the visual analog scale was significantly decreased at 1st week and 4th week after injection, as compared with the time of pre-injection, with mean 5.4 points at the time of pre-injection, 4.05 points at 1st week and 3.55 at 4th week, respectively (p<0.05). McGowan score and SQUNE tended to be decreased after injection, although it did not reach statistical significance (
Fig. 2).
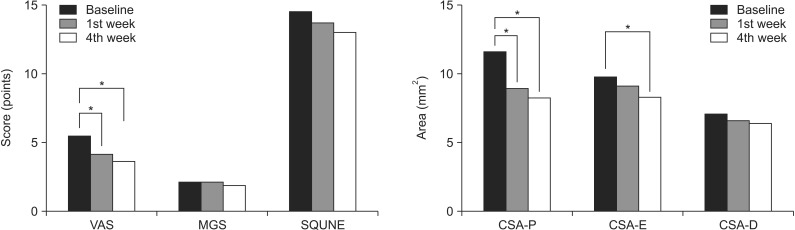 | Fig. 2Symptom scores and morphologic changes were compared at baseline, 1st week, and 4th week after injection. Among symptom scores, visual analog scale (VAS) was significantly decreased at 1st week and 4th week, as compared to baseline. Cross-sectional area (CSA) of ulnar nerve was measured by ultrasonography at 3 points of P, E, and D (P, 2 cm proximal to the epicondyle; E, the level of the epicondyle; D, 2 cm distal to the epicondyle). Comparing with baseline, the CSA-P showed significant decrease at 1st and 4th week. The CSA-E was significantly decreased at 4th week, as compared to baseline. MGS, McGowan classification; SQUNE, self-administered questionnaire of the ulnar neuropathy at the elbow. *p<0.05.
|
The cross-sectional area of the ulnar nerve was measured by US at 2 cm proximal to the epicondyle, the level of the epicondyle, and 2 cm distal to the epicondyle. The cross-sectional area measured at 2 cm proximal to the epicondyle showed significant decreases at the 1st week and 4th week, as compared to the time of pre-injection, with mean 11.50, 8.80, and 8.10 mm2, respectively (p<0.05). The mean cross-sectional areas at the level of the epicondyle were 7.0, 6.5, and 6.3 mm2 at the time of pre-injection, 1st week, and 4th week, respectively. There was a significant size difference in the cross-sectional areas at the level of the epicondyle between the time of pre-injection and 4th week (p<0.05), while significant morphologic change was not observed between the 1st and 4th week. The cross-sectional area measured at 2 cm distal to the epicondyle did not show significant morphologic changes according to the different evaluation times.
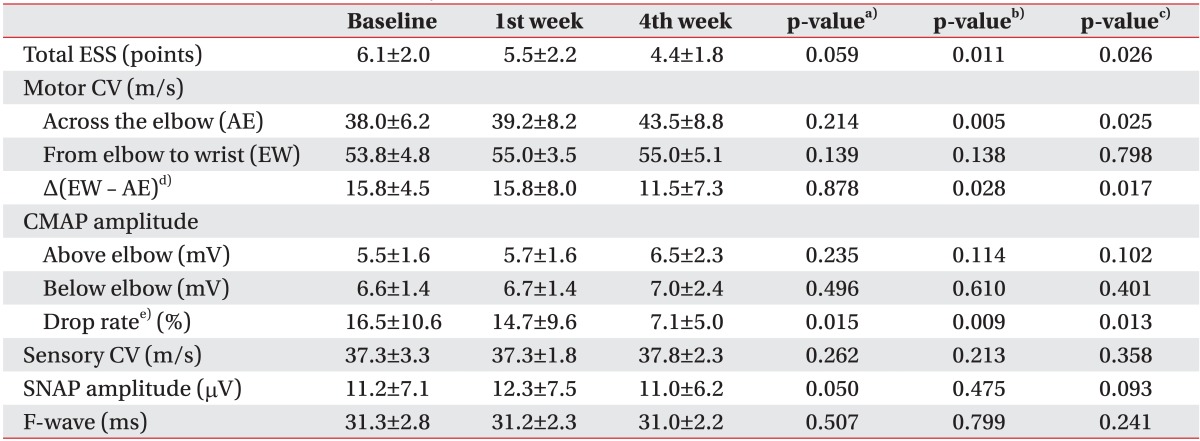
On electrophysiologic analysis of the ulnar nerve, we observed that the electrophysiological severity scale was significantly decreased at the 4th week, as compared to pre-injection and 1st week (p=0.011 and p=0.026, respectively). Among the quantitative parameters of electrophysiological severity scale, motor conduction velocity in the forearm segment from the elbow to the wrist did not show significant changes throughout the study duration. However, motor conduction velocity across the elbow was significantly increased and the difference of motor conduction velocity between the segment of forearm and across the elbow were significantly decreased correspondingly at 4th week, as compared to pre-injection and 1st week, respectively (p=0.005, p=0.025 and p=0.028, p=0.017). The amplitude of CMAP at each point of above and below elbow, showed no significant electrophysiological changes. However, drop rate of the amplitude of CMAP was consistently decreased not only at the 1st but also at the 4th week, (p=0.015 and p=0.013, respectively) (
Table 2).
Table 2
Comparison of electrophysiological severity scale for ulnar nerve and it's components according to the times of baseline, 1st week and 4th week after injection

Go to :

DISCUSSION
The purpose of this study was to determine the clinical applicability of the US-guided injection by the new in-plane approach for the potential treatment of CuTS. The results presented here showed clinically relevant symptomatic, anatomic, and electrophysiological improvements in CuTS patients using US-guided injection by in-plane approach.
There have been few reports on the treatment of steroid injection for CuTS until the mid-2000s [
14,
15]. Because US was not actively used, the early studies performed a blind injection and failed to demonstrate a significant injection effect, as compared to placebo effect or conventional splinting. In response, this generated a controversy related to steroid injection. Recently however, Rampen et al. [
6] briefly reported that US-guided injection using an out-of-plane technique improved symptoms in patients of CuTS, however, statistical verification was not conducted. On the other hand, Alblas et al. [
7] described that there was no significant improvement of morphologic changes through their study on the feasibility of US-guided injection in dispersing steroid near the ulnar nerve. In these studies, the treatment outcomes were different and the injection techniques differed, thus raising concerns about the US-guided injection procedure in CuTS. Kim et al. [
8] used cadaveric specimens to introduce a new in-plane approach and demonstrated that it was a feasible and more accurate cubital tunnel injection technique. They demonstrated the importance of the hydrodissection effect to separate the ulnar nerve from adjacent connective tissues, and suggested that it may disrupt adhesion and alleviate inflammation. Using the real-time US in-plane approach, needle advancement can be visualized throughout the process and the nerve can be accurately targeted through the shortest path. This maximizes the injection accuracy and limits incidental complications [
16].
In the present study, we found that symptom scores and cross-sectional areas measured at 2 cm proximal to the epicondyle tended to decrease with time throughout the study. Interestingly, the recovery time related to morphological improvements did not correspond to the time of electrophysiological improvement. The symptomatic recoveries and morphologic changes appeared before the electrophysiological improvement. The morphological changes began to significantly decrease at the 1st week after injection. On the other hand, electrophysiological improvement was not identified until the 4th week after injection, because the difference of motor conduction velocity between the segment of forearm and across the elbow was decreased at the 4th week, as compared to pre-injection and 1st week. Consistent with our finding, the earlier studies also described the rapid improvement in inflammation and edema in entrapment neuropathy, when steroid injection was applied to the swollen ulnar nerve at the proximal portion of cubital tunnel [
5,
13]. It is estimated that neural regeneration occurs subsequent to the early anti-inflammatory effect of steroid. In fact, an in vivo study reported that regenerative myelination is observed at 3 weeks after nerve damage on glucocorticoid treatment [
17], which may explain why electrophysiological improvement was found at the 4th week after the injection in our study.
The amplitude of CMAP at each point of above and below the elbow, showed no significant electrophysiological changes. However, drop rates of the amplitude of CMAP measured at both 1st week and 4th week were decreased. The improvement of motor conduction velocity and the drop rate of CMAP across the elbow appeared to contribute to the enhancement of overall electrophysiological severity. Because the drop rate of the CMAP suggests the presence of a conduction block, the steroid injection is thought to resolve focal demyelination [
17].
This pilot study was limited by the small number of participants, lack of a control group, and the short-term study period. Nevertheless, some important conclusions were derived from these results. Accurate US-guided injection using the new in-plane approach provided clinical improvements and therapeutic effects, based on improvements in the symptom scale scores, anatomic morphologic changes, and electrophysiological recoveries in patients with CuTS. Our results indicated that the new approach is a safe and accurate non-surgical treatment for patients with CuTS. In future, a large scale and long-term follow-up study, compared with other treatment modalities, will be necessary to determine the effectiveness and efficiency of the new approach to injection.
In conclusion, our study demonstrated that the new maneuver of US-guided injection using in-plane technique might be a safe and effective treatment tool for CuTS in terms of symptomatic, morphologic, and electrophysiological outcomes. We found significant symptomatic, morphological, and electrophysiological improvements in patients with CuTS following the steroid injection. However, the recovery time, as measured by morphological improvements did not correspond with the time of electrophysiological improvement, suggesting that the symptomatic and morphologic improvements may have occurred before electrophysiological improvements.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download