Abstract
Objective
To evaluate the effect of caffeine on balance control of hemiparetic stroke patients, we investigated the difference in postural stability before and after drinking coffee by observing changes in stability index (SI) from posturography.
Methods
Thirty patients with history of stroke and 15 age-matched healthy subjects participated in this study. Effect of group factor (of the control and stroke groups) and treatment factor (pre- and post-drinking of coffee) on SI were tested in three conditions: with eyes opened, with eyes closed, and with a pillow support. The effects of these factors on visual deprivation and somatosensory change of subjects were also tested.
Results
Under all conditions, SI was higher in the stroke group than in the control group. Under eyes-open condition, the treatment factor was not statistically significant. Under eyes-closed condition, the interaction between group and treatment factor was statistically significant. After the subjects drank coffee, SI in the control group was increased. However, SI in the stroke group was decreased. Under pillow-supported condition, the interaction between group and treatment factor appeared marginally significant. For visual deprivation effect, the interaction between treatment and group factor was statistically significant. After caffeine consumption, the visual deprivation effect was increased in control group but decreased in the stroke group. For somatosensory change effect, the interaction between group and treatment factor was not statistically significant.
Caffeine is a xanthine alkaloid compound commonly found in coffee, soft drinks, green tea, cocoa, chocolate, and medicine [1]. An appropriate amount of caffeine as a central nervous system stimulant has beneficial effects in reducing physical fatigue and restoring alertness [2]. Many previous studies have reported that caffeine also could enhance physical endurance and reduce reaction time [3,4,5]. In addition, recent studies have found that consumption of caffeine could improve the motor manifestations in Parkinson disease [6,7,8,9,10]. However, some studies have reported inconsistent balance control results regarding the effect of caffeine on physical performance. Norager et al. [3] studied the effect of caffeine on physical performance in healthy elderly subjects. Their results showed that subjects' endurance was improved. However, their postural sway was increased after their caffeine intake. Lee [11] performed a study using 40 healthy men in their 20s and reported that caffeine did not significantly improve their maintenance of their standing balance. The study by Enriquez et al. [12] of 23 healthy adults showed that caffeine did not significantly stabilize subjects' posture.
Postural stability is maintained by physical performance ability and integration of sensory inputs, such as somatic, visual, and vestibular sensors. However, stroke patients have various sensory impairments that lead to motor control failure and reduced balance control. Thus, they not only have problems with walking and movement but also have high risk of falls. Although stroke patients without medical restrictions are allowed to consume caffeine, the effect of caffeine on postural stability in stroke patients has not yet been sufficiently studied. Therefore, the objective of this study was to determine the effect of caffeine on postural stability in stroke patients compared to age-matched healthy elderly subjects. By comparing their conditions before and after they drank caffeine beverage, we investigated how the average amount of caffeine consumed by Koreans would influence their static postural control depending on visual and somatic sensory inputs via changes in their stability index (SI) using posturography (Tetrax; Sunlight Medical Ltd., Ramat Gan, Israel).
Of patients who were admitted to the inpatient and outpatient rehabilitation department and other departments, 30 hemiparetic patients with a history of stroke and 15 age-matched healthy subjects were enrolled in this study. Most of the stroke group was in a state refraining from coffee intake before the onset of stroke. Only 16.6% (5 out of 30) sustained coffee intake about 1-2 cups per day, the same as before the stroke. Most of the control group habitually consumed a coffee daily. In general, addiction was defined as intake behavior of taking food or medicine continuously due to physical and mental disabilities [13,14]. There was no subject addicted to food containing caffeine including coffee. Before experiments, regular diet was allowed. However, caffeinated coffee and tea were prohibited.
They were able to independently stand for 10 minutes without using any assistive device, such as a cane or a walker. Only subjects who frequently drank coffee or used to drink it and who had never been sensitive to caffeine were enrolled in this study. Subjects who had one of the following diseases or conditions were excluded: 1) could not stand independently; 2) had never drank coffee; 3) had complained of side effects of caffeine; 4) had a disorder that could affect their postural stability, such as deformity in their lower extremities, pain, or history of neurologic injury; and 5) had a history of Parkinson disease or was taking dopaminergic medication. All subjects voluntarily participated in this study which was approved by the Institutional Review Board of the Veterans Health Service Medical Center.
To evaluate the effect of caffeine intake, we used instant 'K' coffee. The caffeine amount (around 73.41 mg) was similar to that of other instant coffees mostly consumed by Koreans. No additive such as sugar, milk products, or artificial sweeteners was added to the coffee. Before and after caffeine intake, postural stability was estimated with Tetrax (Sunlight Medical Ltd.), a static posturography device. The postural stability was measured at any time after 40 minutes from the start of the test, which included the preparation time and breaks, because blood concentration of caffeine was reported to be the highest 40-60 minutes after oral administration [15,16].
For the evaluation of postural stability using Tetrax, subjects had to be prepared. They took off their shoes, positioned their feet on the force plates, held support bars, and stood in a comfortable position. After taking their hands off the bar and standing at attention, the subjects were asked to maintain their position during the test as much as possible. The test was carried out in eight different conditions in the following order: with the subjects' eyes open, with their eyes closed, with their eyes open while an elastic plate (31×12 cm) was placed under their feet to make the ground unstable (i.e., the pillow-supported condition), and with their eyes closed while the elastic plate was placed under their feet. They had four different postures while turning their head from side to side. The test was repeated if the subject could not keep his or her feet on the force plate or maintain his or her position as recommended [17,18]. We let the subjects take a 10-minute break before the test and midway through the test to reduce their physical fatigue and minimize the possibility of changes in their postural stability. More than two persons, including the examiners, were always on hand to prevent falls, and they helped the subjects when needed. The Berg balance (BBS) test and Timed up and go (TUG) test used for clinical assessments of balance and mobility performance were carried out before and after coffee intake. Tetrax is a device for measuring the ratio of the weight loaded on the fore part and rear part of both feet by installing piezoelectric sensors on four force plates. The sampling rate was 32 times per second (Hz). The ratios from each force plate were measured for 32 seconds. Accordingly, the total number of data from one condition was 4096 (4×1024, i.e., 4 force plates×32 times×32 seconds). Based on these data, the SI, weight distribution index, synchronization index, fall index, Fourier transformation, and frequency analysis were provided. The SI used in this study was calculated using the following equation to provide average volatility of the weight distribution measured on the four force plates:
In the preceding equation, j indicated the force plate, i the sample number, and F the percentage (%) of the body weight measured with force plates. Therefore, an increase in the SI would mean increases in the average volatility of the distribution of the weight loaded on the four force plates and in the instability. Among the eight different conditions measured via Tetrax posturography, the SI was analyzed in three conditions related to visual and somatosensory information: 1) with the eyes open; 2) with the eyes closed; and 3) with a pillow support. The SI difference between the eyes-open and eyes-closed conditions (SI_V) and the SI difference between the eyes-open and pillow-supported conditions (SI_S) were defined as dependent variables.
A t-test was conducted to compare the characteristics of the stroke and control groups. Standard deviation and the average of dependent variables were calculated as the representative values in 12 situations (2×2×3=12) as follows: group factor (control vs. stroke groups)×treatment factor (pre-caffeine vs. post-caffeine)×experimental situation (eyes open, eyes closed, and pillow-supported). Linear mixed model (LMM) was used to test the effect of the factors. The group factor, which was between the subject factor, and the treatment factor, which corresponded to the within subject factor, were defined as the fixed factors. Subjects were defined as the random factor. Random intercept by subject and random slope by the treatment effect for dependent variables were calculated. Fixed factor effects and their interactions were tested. Fixed effect was tested by comparing null model without fixed effect and full model with fixed effect. Due to difference in the number of samples of the control and stroke groups, data were unbalanced repetitive measurements. Open software R ver. 2.15.0 (R Core Team, http://www.r-project.org) and lme4 package [19] were used for LMM analysis.
All subjects were men at age of 64.6±11.51 years in the control group and 69.1±8.60 years in the stroke group. There was no statistically significant difference in age between the two groups. The average disease duration of stroke group was 44.33 months. The BBS scale in the stroke group was significantly (p<0.01) lower than that in the control group (38.38±10.75 vs. 54.66±2.59). The Fugl-Meyer score and the Mini-Mental Status Examination score of the stroke group were 76.33±19.22 and 22.72±6.12, respectively.
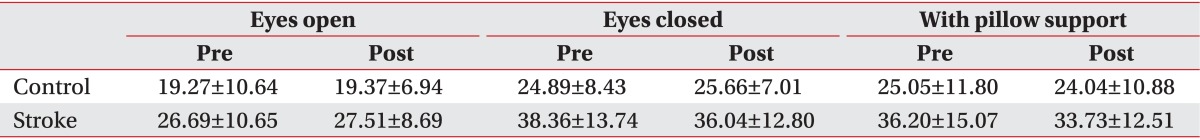
The average and standard deviation of SI before and after coffee intake in the control and stroke groups are summarized in Table 1.
At the eyes-open condition, the group factor had a statistically significant effect on the SI (χ2(1)=7.70, p<0.01), with the stroke group showing a larger SI compared to the control group. The treatment factor did not have a statistically significant effect on the SI (χ2(3)=2.66, p=0.44). The interaction between the treatment factors and the group factors was not statistically significant (χ2(1)=1.04, p=0.30) (Table 1).
The interaction between the group factor and the treatment factor was statistically significant (χ2(3)=3.74, p=0.05). After coffee intake, SI was increased in the control group but decreased in the stroke group compared to SI before coffee intake (Fig. 1). The group factor had a statistically significant effect on the SI at the eyes-closed condition (χ2(1)=9.56, p<0.01) (Table 1).
When changes were induced in the somatosensory inputs by changing the base of support in the eyes-open condition, the group factor was statistically significant (χ2(1)=8.66, p<0.01), with SI higher in the stroke group compared to the control group (Table 1). The treatment factors (χ2(1)=7.04, p=0.07) and the interaction between the group and treatment factors (χ2(3)=8.25, p=0.08) were marginally significant.
The representative values (average and standard deviation) of the SI_V and SI_S are summarized in a cross-table (Table 2) by group and treatment factor. To identify the group effect on SI_V, the effect of group factor was tested using data before coffee intake. The group factor showed a statistically significant effect (χ2(1)=3.81, p=0.05). The SI_V was 5.60±2.69 (standard error [SE]) in the control group. The difference between the control and stroke groups was 6.22±13.19 (SE) in the LMM analysis, indicating that the subjects in the stroke group became more unstable after they closed their eyes.
To analyze the effect of treatment factor on SI_V, the group and treatment factors and their interaction were tested using data before and after coffee intake. The interaction was statistically significant (χ2(1)=4.37, p=0.03). Although SI_V was increased in the control group, it was decreased in the stroke group after coffee intake (Table 2, Fig. 2).
The effect of group factors on SI_S was tested using data before coffee intake to figure out the difference in the somatosensory change effect between the control and stroke groups. The SI_S before coffee intake was 5.78±2.76 (SE) in the control group. The difference in the SI_S values of the two groups was 3.78±3.27 (SE), with an increase in the stroke group. However, the group factors did not show a statistically significant effect (χ2(1)=1.39, p=0.23) on SI_S.
The group and treatment factors and their interaction were tested using data before and after coffee intake to analyze the effect of caffeine intake on SI_S. The interaction of the group and treatment factors was not statistically significant (χ2(1)=0.14, p=0.70). The group factor was not statistically significant either (χ2(1)=1.27, p=0.25). The treatment factor was only marginally significant (χ2(3)=6.33, p=0.09). The SI_V was decreased after coffee intake (Table 2, Fig. 2).
The treatment and situation factors and their interaction were observed in the control and stroke groups to find out if treatment factors according to caffeine intake would differ in different experiment situation. While the interaction was not statistically significant in the control group (χ2(2)=2.47, p=0.29), it was statistically significant in the stroke group (χ2(3)=6.20, p=0.04).
Balance control is the ability to maintain the center of the body mass on the base of support during static and dynamic movements. Posture control is a function that maintains balance and appropriate relations between body segments by considering the relation between a body and an object in the environment [20].
Sensory inputs play key roles in maintaining balance. However, somatic, visual, and vestibular sensory information must be integrated [21]. These sensory information act independently during balance control, sometimes disturb each other's actions, thereby degrading their function in maintaining balance. Accordingly, the capacity to choose, analyze, and integrate appropriate sensory information is important [22].
Stroke patients have sensory loss, muscle weakness, motor control dysfunction, abnormal increase in muscle tension, and changes in muscle fiber biomechanical properties in the upper and lower limbs contralateral to the damaged hemisphere. Thus, they are at high risk of falls due to their reduced postural stability [23]. In this study, the SIs analyzed in the eyes-open, eyes-closed, and pillow-supported conditions were higher in the stroke group compared to the control group. This result is consistent with previous studies [24].
With pre-treatment data, SI in the eyes-open condition had a moderate correlation with the Clinical Berg Balance Test (BBS) in the stroke group (r=-0.43, p=0.04; not presented in the results). However, the SI and the TUG test did not show a significant correlation in the stroke group (not presented in the results). These results might have stemmed from the composition of items in the BBS test including the static and dynamic balance and the functions related to balance and walking in the TUG test.
Among various sensory inputs, visual sensory inputs have the most important role in our posture control [25,26]. Novak and Deshpande [27] reported that people heavily rely on visual inputs instead of vestibular information for locomotor control during obstacle crossing. Insufficient visual information could be a risk factor of imbalance or inability. There have been reports that hemiplegic patients, after a stroke, tended to be too dependent on visual inputs, and their balance control and walking ability improved after training with control of visual information [28,29].
In this study, visual deprivation effect (SI_V) was observed in both the control and stroke groups from the eyes-open condition to the eyes-closed condition. Visual deprivation effect was confirmed to be higher in the stroke group compared to the control group. These results were consistent with results of previous studies that reported more visual information used by stroke patients for balance control.
In balance and posture control, visual sensory information plays an important role. However, it is not the only player in balance and posture control because we can also keep standing even in a dark room or with our eyes closed. Visual sensory inputs themselves cannot distinguish our body movements from other movements around us. In addition, our brains can misinterpret visual information. Therefore, somatic, vestibular, and visual sensory information all play important roles in postural stability. Somatosensory receptors are located in our joints, ligaments, muscles, and skin. They transfer proprioceptive information with respect to the length of our muscles, our muscle tension, and the locations of our joints [30]. Most stroke patients experience disturbances in their acquisition of location information in their body due to proprioception degradation, leading to motor control dysfunction and decrease in their movement effectiveness. These phenomena could result in serious restrictions to daily activities [31]. The proprioception degradation in stroke patients is associated with their trunk control ability [32,33]. In this study, we observed subjects with their eyes open after the ground was replaced with a light material to estimate changes in their balance control ability based on somatosensory changes. The somatosensory change effect was greater in the stroke group than in the control group. However, the difference was not statistically significant because the visual sensory dependency was increased but the somatosensory dependency was decreased in the stroke patients.
Caffeine absorbed in the body mainly acts as a sympathetic and central nervous system stimulant, especially in the cerebral cortex. It stimulates our sympathetic nervous system by releasing dopamine, which improves our physical performance. Caffeine also increases neuron firing in our brain. If our pituitary gland senses these activities, our adrenal glands will release adrenaline hormones. Because caffeine increases dopamine release in our brain, feelings of well-being, positive mood, and good physical performance are followed [34,35,36]. Dopamine is well-known to reduce our reaction time and improve our physical performance. Ruscher et al. [37] reported that levodopa treatment improved functional recovery. However, the effect of dopamine therapy on postural stability is controversial. Nova et al. [38] reported that dopaminergic medication improved postural stability measured by BBS test or motor subscale of the Unified Parkinson Disease Rating Scale (UPDRS-III). However, dopaminergic medication did not improve balance impairment in Parkinson disease patients. Based on these, it was suggested that non-dopaminergic lesions also had significant role in the pathophysiology of postural abnormalities [39,40].
The results of this study showed that treatment factors with respect to caffeine intake of healthy subjects were not significant in all situations, including the eyes-open, eyes-closed, and changed base of support conditions. Our result was consistent with that of previous studies that reported caffeine intake did not affect postural stability, suggesting that an increase in dopamine due to caffeine might not improve postural stability. The treatment factor with respect to caffeine intake of stroke patients presented interaction effects with the situation factor, suggesting that caffeine intake in stroke patients may affect not only the motor output but also the integration of sensory information. There was an interaction effect on visual deprivation effect (SI_V) between the group and treatment factors. This indicates that caffeine consumption has different effects on the stroke group and the control group, suggesting that drinking coffee can moderate reduction in balance control ability due to visual deprivation, especially in stroke patients. Therefore, caffeine intake might improve balance control function with somatosensory inputs. In this study, we found how usual amount of caffeine consumed by people influenced postural stability using somatosensory function through the reduction of visual deprivation effect in stroke patients. Therefore, this study provided principal data for the effect of caffeine on balance control.
This study has several limitations. First, it has a limitation in its physiological analysis of the change in the neurotransmitter function due to other ingredients in instant coffee beside caffeine. Second, less caffeine was used in this study than used in previous studies. Additional studies with double-blinded design on postural stability changes according to different amounts of caffeine and the placebo effect should be considered in the future. Third, only static balance was measured in this study. Further studies on dynamic balance control highly related to daily activities should also be performed in the future. Fourth, we did not classify stroke patients according to the severity or location of their lesions. Fifth, no female patient was enrolled in this study due to the composition of the patients in the hospital. Finally, most patients in this study were taking various medicines which might have complex interactions with caffeine. However, we could not control their intake of such medicines.
In conclusion, this study investigated the postural stability with SI from posturography (Tetrax) after caffeine intake in stroke patients compared to healthy controls under three conditions with different sensory inputs related to balance control. The SI was larger in stroke patients than in the control group, indicating that stroke patients were less stable when standing. We also found that the visual sensory dependency of stroke patients was high because the visual deprivation effect was more prominent than the somatosensory change effect. The visual deprivation effect in stroke patients was decreased after drinking coffee. Accordingly, we confirmed that the ability of balance control using somatosensory input after caffeine intake was improved. Therefore, drinking coffee causing an elevated dopamine level is capable of not only improving motor function by reducing reaction time but also improving balance control ability related to somatosensory function. Further studies are required to exclude the aforementioned confounding factors from the limitations of this study.
References
1. Tunnicliffe JM, Erdman KA, Reimer RA, Lun V, Shearer J. Consumption of dietary caffeine and coffee in physically active populations: physiological interactions. Appl Physiol Nutr Metab. 2008; 33:1301–1310. PMID: 19088792.

2. Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002; 40:1243–1255. PMID: 12204388.

3. Norager CB, Jensen MB, Madsen MR, Laurberg S. Caffeine improves endurance in 75-yr-old citizens: a randomized, double-blind, placebo-controlled, crossover study. J Appl Physiol (1985). 2005; 99:2302–2306. PMID: 16081625.

4. Santos VG, Santos VR, Felippe LJ, Almeida JW Jr, Bertuzzi R, Kiss MA, et al. Caffeine reduces reaction time and improves performance in simulated-contest of taekwondo. Nutrients. 2014; 6:637–649. PMID: 24518826.

5. Souissi M, Abedelmalek S, Chtourou H, Atheymen R, Hakim A, Sahnoun Z. Effects of morning caffeine' ingestion on mood States, simple reaction time, and short-term maximal performance on elite judoists. Asian J Sports Med. 2012; 3:161–168. PMID: 23012635.

6. Prediger RD. Effects of caffeine in Parkinson's disease: from neuroprotection to the management of motor and non-motor symptoms. J Alzheimers Dis. 2010; 20(Suppl 1):S205–S220. PMID: 20182024.

7. Altman RD, Lang AE, Postuma RB. Caffeine in Parkinson's disease: a pilot open-label, dose-escalation study. Mov Disord. 2011; 26:2427–2431. PMID: 21953603.

8. Factor S, Mark MH, Watts R, Struck L, Mori A, Ballerini R, et al. A long-term study of istradefylline in subjects with fluctuating Parkinson's disease. Parkinsonism Relat Disord. 2010; 16:423–426. PMID: 20338800.

9. Hauser RA, Cantillon M, Pourcher E, Micheli F, Mok V, Onofrj M, et al. Preladenant in patients with Parkinson's disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol. 2011; 10:221–229. PMID: 21315654.

10. Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, Moscovich M, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012; 79:651–658. PMID: 22855866.

11. Lee JW. Effects of caffeine of coffee on fine motor, gross motor and balance in healthy adult male [dissertation]. Daejeon: Chungnam National University;2007.
12. Enriquez A, Sklaar J, Viirre E, Chase B. Effects of caffeine on postural stability. Int Tinnitus J. 2009; 15:161–163. PMID: 20420341.
13. Angres DH, Bettinardi-Angres K. The disease of addiction: origins, treatment, and recovery. Dis Mon. 2008; 54:696–721. PMID: 18790142.

14. Marlatt GA, Baer JS, Donovan DM, Kivlahan DR. Addictive behaviors: etiology and treatment. Annu Rev Psychol. 1988; 39:223–252. PMID: 3278676.

15. Ivy JL, Costill DL, Fink WJ, Lower RW. Influence of caffeine and carbohydrate feedings on endurance performance. Med Sci Sports. 1979; 11:6–11. PMID: 481158.
16. Graham TE. Caffeine and exercise: metabolism, endurance and performance. Sports Med. 2001; 31:785–807. PMID: 11583104.
17. Kim CR, Chun MH, Lee GA. Assessments of balance control using tetra-ataxiametric posturography. J Korean Acad Rehabil Med. 2009; 33:429–435.
18. Kim BR, Choi KH, Chun MH, Lee MC, Chung SJ, Jang KW. Evaluation of balance control in patients with idiopathic Parkinson's disease using tetra-ataxiometric posturography. J Korean Acad Rehabil Med. 2009; 33:538–546.
19. Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008; 59:390–412.

20. Gjelsvik BE. The Bobath concept in adult neurology. Stuttgart: Thieme;2008. p. 19–21.
21. Di Fabio RP, Emasithi A. Aging and the mechanisms underlying head and postural control during voluntary motion. Phys Ther. 1997; 77:458–475. PMID: 9149758.

22. Bonan IV, Guettard E, Leman MC, Colle FM, Yelnik AP. Subjective visual vertical perception relates to balance in acute stroke. Arch Phys Med Rehabil. 2006; 87:642–646. PMID: 16635626.

23. Kerrigan DC, Karvosky ME, Riley PO. Spastic paretic stiff-legged gait: joint kinetics. Am J Phys Med Rehabil. 2001; 80:244–249. PMID: 11277129.
24. Kohen-Raz R. Application of tetra-ataxiametric posturography in clinical and developmental diagnosis. Percept Mot Skills. 1991; 73:635–656. PMID: 1766798.

25. Massion J, Amblard B, Assaiante C, Mouchnino L, Vernazza S. Body orientation and control of coordinated movements in microgravity. Brain Res Brain Res Rev. 1998; 28:83–91. PMID: 9795153.

26. Paulus WM, Straube A, Brandt T. Visual stabilization of posture: physiological stimulus characteristics and clinical aspects. Brain. 1984; 107(Pt 4):1143–1163. PMID: 6509312.
27. Novak AC, Deshpande N. Effects of aging on whole body and segmental control while obstacle crossing under impaired sensory conditions. Hum Mov Sci. 2014; 35:121–130. PMID: 24746603.

28. Bonan IV, Yelnik AP, Colle FM, Michaud C, Normand E, Panigot B, et al. Reliance on visual information after stroke. Part II: Effectiveness of a balance rehabilitation program with visual cue deprivation after stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004; 85:274–278. PMID: 14966713.

29. Marigold DS, Eng JJ. The relationship of asymmetric weight-bearing with postural sway and visual reliance in stroke. Gait Posture. 2006; 23:249–255. PMID: 16399522.

30. Woollacott MH, Shumway-Cook A, Nashner LM. Aging and posture control: changes in sensory organization and muscular coordination. Int J Aging Hum Dev. 1986; 23:97–114. PMID: 3557634.

32. Ryerson S, Byl NN, Brown DA, Wong RA, Hidler JM. Altered trunk position sense and its relation to balance functions in people post-stroke. J Neurol Phys Ther. 2008; 32:14–20. PMID: 18463551.

33. Goldberg A, Hernandez ME, Alexander NB. Trunk repositioning errors are increased in balance-impaired older adults. J Gerontol A Biol Sci Med Sci. 2005; 60:1310–1314. PMID: 16282565.

34. Solinas M, Ferre S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci. 2002; 22:6321–6324. PMID: 12151508.

35. Kaasinen V, Aalto S, Nagren K, Rinne JO. Dopaminergic effects of caffeine in the human striatum and thalamus. Neuroreport. 2004; 15:281–285. PMID: 15076753.

36. Garrett BE, Griffiths RR. The role of dopamine in the behavioral effects of caffeine in animals and humans. Pharmacol Biochem Behav. 1997; 57:533–541. PMID: 9218278.

37. Ruscher K, Kuric E, Wieloch T. Levodopa treatment improves functional recovery after experimental stroke. Stroke. 2012; 43:507–513. PMID: 22096034.

38. Nova IC, Perracini MR, Ferraz HB. Levodopa effect upon functional balance of Parkinson's disease patients. Parkinsonism Relat Disord. 2004; 10:411–415. PMID: 15465397.

39. Bloem BR, Beckley DJ, van Dijk JG, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease. Mov Disord. 1996; 11:509–521. PMID: 8866492.

40. Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson's disease: the adrenergic hypothesis and the locus coeruleus. Expert Rev Neurother. 2009; 9:279–290. PMID: 19210201.

Fig. 1
Changes of stability index after drinking coffee. (A) With the eyes open, (B) with the eyes closed, and (C) with pillow support. Dashed line represents the stroke group; solid line represents the control group. Pre, before drinking a caffeine beverage; post, after drinking a caffeine beverage.
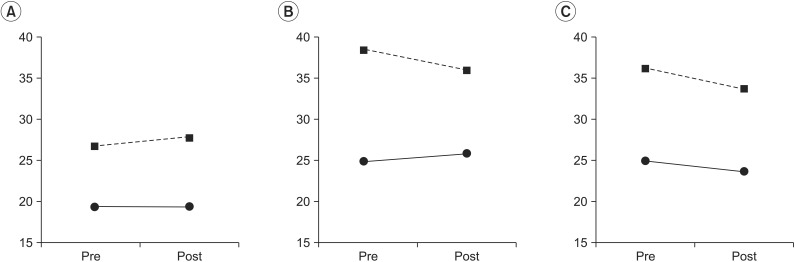
Fig. 2
Changes of stability index difference after drinking coffee. (A) Visual deprivation and (B) somatosensory change. Dashed line represents the stroke group; solid line represents the control group. Pre, before drinking coffee; post, after drinking coffee; visual deprivation, the difference between the stability index in the eyes-closed condition and that in the eyes-open condition; somatosensory change, the difference between the stability index in the pillow-supported condition and that in the eyes-open condition.

Table 2
Visual deprivation effect and somatosensory change effect for each cell of the 2×2 cross-table by group and treatment
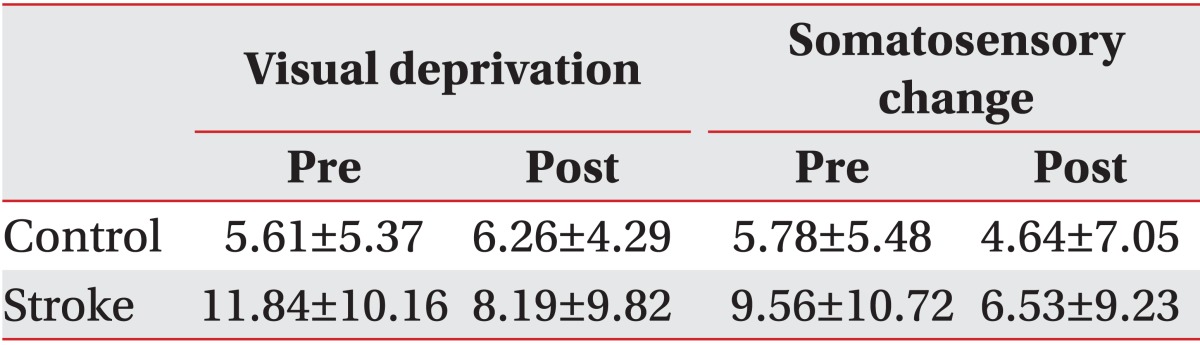
The values are averages across trials and subjects and standard deviations of the differences in the stability indices from Tetrax.
Visual deprivation, difference between the stability index in the eyes-closed condition and that in the eyes-open conditions; somatosensory change, difference between the stability index in the pillow-supported condition and that in the eyes-open condition; pre, before drinking coffee; post, after drinking coffee.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


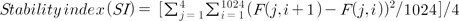
 XML Download
XML Download