Abstract
Objective
To assess the efficacy of trigger point injection into brachialis muscle for rotator cuff disease patients with upper arm pain.
Methods
A prospective, randomized, and single-blinded clinical pilot trial was performed at university rehabilitation hospital. Twenty-one patients clinically diagnosed with rotator cuff disease suspected of having brachialis myofascial pain syndrome (MPS) were randomly allocated into two groups. Effect of ultrasound (US)-guided trigger point injection (n=11) and oral non-steroidal anti-inflammatory drug (NSAID) (n=10) was compared by visual analog scale (VAS).
Results
US-guided trigger point injection of brachialis muscle resulted in excellent outcome compared to the oral NSAID group. Mean VAS scores decreased significantly after 2 weeks of treatment compared to the baseline in both groups (7.3 vs. 4.5 in the injection group and 7.4 vs. 5.9 in the oral group). The decrease of the VAS score caused by injection (ДVAS=-2.8) was significantly larger than caused by oral NSAID (ДVAS=-1.5) (p<0.05).
Rotator cuff disease including tendon tearing, prevalent in middle-aged individuals, is one of the most common causes of shoulder pain [1]. Treatments for rotator cuff lesions without complete tears are mainly conservative [2]. Among various options, subacromial injection of anesthetics or corticosteroids and oral non-steroidal anti-inflammatory drugs (NSAIDs) are frequently used to treat patients with persistent symptoms after rehabilitative therapy [3].
Myofascial pain syndrome (MPS) is considered as one of the possible etiologies for shoulder pain. However, MPS rarely occurs as a primary origin of pain [4,5]. Considerable evidences suggest that MPS is caused by or related to a lesion in another soft tissue, such as rotator cuff disease [5,6]. Rather than being a primary generator of shoulder pain, MPS is accompanied with other shoulder lesions, such as rotator cuff disease which might overlap with the symptoms of shoulder lesions and aggravate the pain.
Previous studies on MPS of shoulder region usually focused on the shoulder girdle muscles or muscles directly attached to the shoulder joint [7,8]. The trapezius, infraspinatus, supraspinatus, subscapularis, teres minor and major, deltoid, pectoralis, biceps brachii, and triceps muscles are all possible sources of shoulder pain in rotator cuff disease [7]. However, in our clinical experience, a significant number of patients with rotator cuff disease also have the brachialis muscle-originated upper arm pains.
The brachialis muscle, along with biceps brachii muscle, is one of the main elbow flexors. Unlike biceps brachii, which also participates in elbow supination, the brachialis muscle works only in elbow flexion [9]. When the biceps brachii muscle is affected by rotator cuff diseases, the brachialis muscle may be under more loading pressure and overused on elbow flexion. Therefore, compared to those without shoulder rotator cuff disorders, patients with rotator cuff pathology may be more prone to the development of myofascial trigger points (MTrPs).
The common conservative treatment of rotator cuff disease includes physical therapy and anti-inflammatory therapy (either systemic or local) [10]. However, patients with rotator cuff disease combined with suspected MPS on the brachialis muscle do not respond well to conventional conservative therapy that only targets rotator cuff tendon itself. Therefore, we hypothesized that for patients with rotator cuff disease who do not respond well to therapy focusing on the primary shoulder lesion, the MPS of the brachialis muscle could be considered as an alternative pain generator, and that ultrasound (US)-guided trigger point injection could be useful to diagnose the combined MPS on the brachialis and to treat their pain. This study applied a novel US-guided trigger point injection of the brachialis for those patients with rotator cuff disease who did not respond to the subacromial injections. The study aimed to investigate the therapeutic effectiveness of US-guided trigger point injection for active MTrPs in the brachialis muscles. The objectives of this study were 1) to determine whether the newly approached US-guided trigger point injection of the brachialis could be effective for pain control in patients with chronic rotator cuff disease combined with MPS on the brachialis and 2) to investigate whether the brachialis was the cause of upper arm pain in some patients with rotator cuff disease.
Of patients with rotator cuff disease who visited the outpatient clinic of a university hospital between March and August 2013, those who met the following criteria were included in this study 1) were diagnosed with rotator cuff disease from a tendinosis to a partial-thickness tear of the supraspinatus based on sonographic examination or magnetic resonance arthrography; 2) had proximal upper arm pain below the shoulder joint in the affected shoulder side; 3) had a pain score measured by the visual analog scale (VAS) greater than 5 (on a numeric scale of 0-10); 4) no weakness on resisted testing of musculotendinous units of the rotator cuff; 5) had no less than 20% reduction in proximal upper arm pain with subacromial injections of local anesthetics and steroids; and 6) had diagnosis of MPS in the brachialis muscle. Exclusion criteria were 1) presence of other obvious pathology for the rotator cuff pain including fracture or rheumatic diseases; 2) MPS in muscles other than the brachialis muscle; 3) neurologic shoulder/axillary pain if the patient had a history of posterior neck pain, 4) signs and symptoms of neuropathy in the affected upper limbs; 5) a history of other treatments for upper arm pain, such as NSAID use or physical therapy, within two weeks before subacromial injections; 6) a history of subacromial injection and/or trigger point injection within 3 months; 7) previous history of adverse effect of lidocaine or steroid; 8) gastrointestinal discomfort with NSAIDs; 9) presence of an unstable medical condition or a known uncontrolled systemic disease; and 10) any conditions or situations that might place the patient at significant risk during the study.
Diagnosis of an active MPS was based on the modified criteria described by Travell and Simons [11]: 1) tender spots in the brachialis; 2) a typical pattern of referred pain is elicited when tender spots are compressed; 3) restricted range of motion; and 4) local twitch response (LTR). Tenderness could be elicited in the brachialis muscle by palpating the muscle between the biceps brachii and triceps muscles. However, the criterion of a palpable or visible LTR on snapping palpation at the most sensitive spot in the taut band was excluded because it would be impossible to observe LTRs in every case due to the difficulty in palpating the whole brachialis muscle. Instead, we elicited and assessed LTRs during US-guided trigger point injections [12].
This study was approved by the Institutional Review Board and human subjects review committee before the study began. Written informed consent was obtained from all participants after they were briefed on the purpose and procedures of the study.
The present pilot study was designed as a prospective, randomized, and single-blinded clinical pilot trial that compared trigger point injection of the brachialis muscle with oral NSAIDs. First, patients who had painful arc and/or impingement sign were diagnosed with supraspinatus tendon disease, such as a tendinosis or a partial-thickness tear by ultrasonography or magnetic resonance arthrography. Among these patients, we selected those with proximal arm pain on the same affected side of the shoulder pathology. Assuming the arm pain as 'referred pain from rotator cuff disease', we performed US-guided subacromial injection (a mixture of 4 mL of 0.5% lidocaine and 1 mL of 40 mg of triamcinolone), and those with <20% of pain reduction 2 weeks after a subacromial injection were finally included in the study. These patients were randomly grouped into the oral NSAID or trigger point injection groups. Oral NSAID group received naproxen 500 mg twice a day for 2 weeks. Injection group received one US-guided trigger point injection into the brachialis muscle.
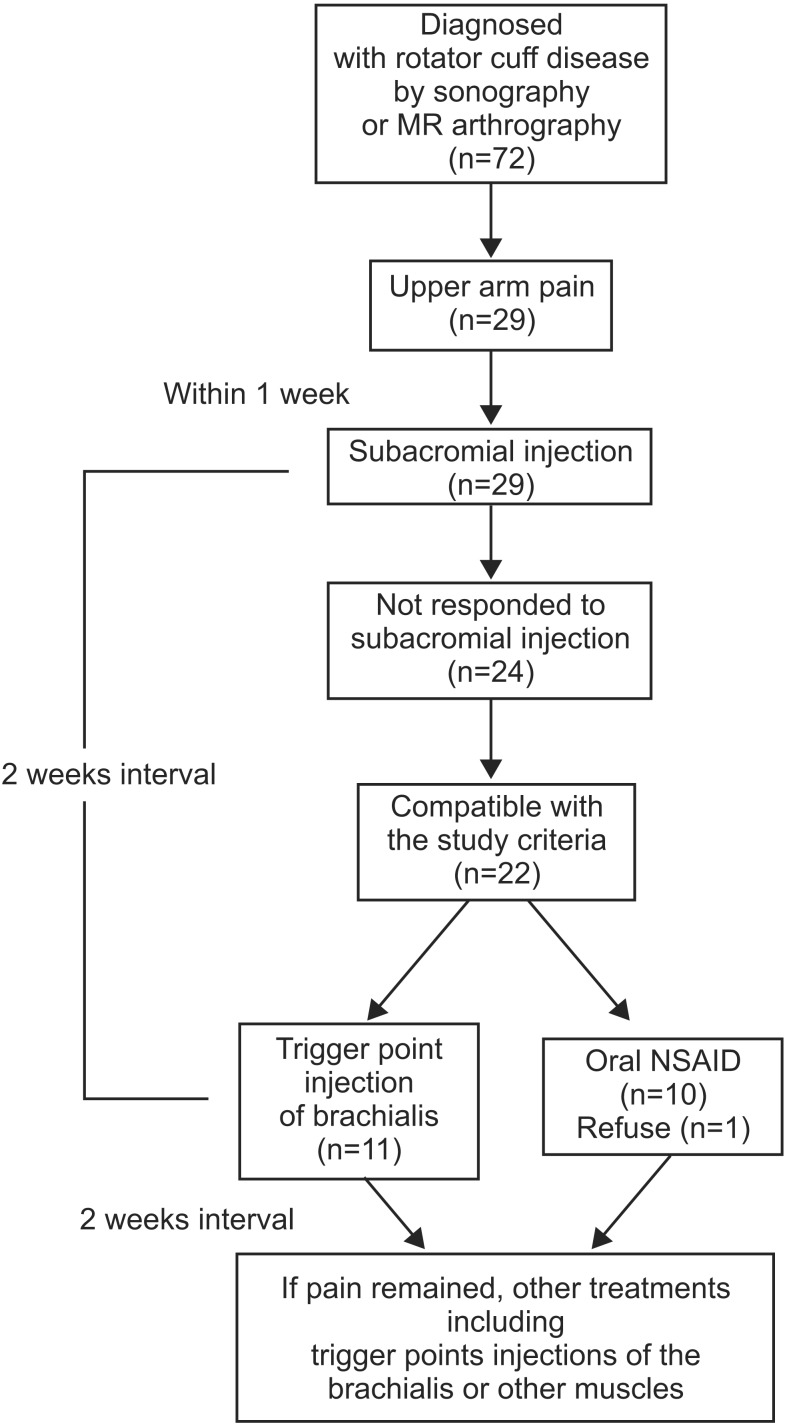
One physiatrist who was not blinded examined all patients who were referred to the clinic for eligibility. The investigator who evaluated the outcome measures was blinded to the group allocation throughout the study, although the physiatrist who performed all injections and supervised the consumption of oral NSAIDs and the participants were not blinded. Another trigger point injection of the brachialis or other muscles along with other oral analgesics and physical therapy were performed if pain reduction was not satisfactory after 2 weeks. Trigger point injections were performed at a 2-week interval. For the purpose of this study, we limited the study period to 2 weeks. The comparison of the final outcome was executed 2 weeks after the first injection in the injection group, and 2 weeks after daily medication of NSAID in the oral medication group. The study design is illustrated in Fig. 1.
US-guided trigger point injection methods, previously reported for lower back and deep pelvic muscles, have been modified for MTrPs in brachialis [12,13]. We performed B-mode, real-time ultrasonography with sterile methods using an Accuvix V10 ultrasound machine (Medison, Seoul, Korea) interfaced with a 5- to 12-MHz linear array transducer around the targeted muscle. A physiatrist with more than 7 years of experience in musculoskeletal ultrasonography carried out US-guided injection procedures.
For ultrasound examination and injection procedures, the patient was positioned lying on the lateral side with the affected side up. The upper arm was placed tightly to the trunk to minimize motion during injection (Fig. 2). The biceps was relaxed with the forearm pronated.
We assessed the borderline between the brachialis and biceps brachii from the lateral aspect of the upper arm and marked the maximal tender point on the skin by palpating the muscle. The proximal attachment of the brachialis is located around the deltoid tuberosity of the humerus. To scan the brachialis muscle properly, the probe started from the deltoid tuberosity distally to the distal part of upper arm. By positioning the probe on the marking point and turning the probe to show the best view, we obtained the transverse image of the brachialis muscle (Fig. 3). In this position, the biceps brachii was located above the brachialis on the US image. In case of multiple tender spots in one muscle, US-scanning and injection procedures were repeated for all tender spots. Color Doppler images were used to avoid neurovascular bundle. The Doppler setting changed at the level where vascular structures were optimally visualized in each subject. Under US-guidance, a 23-gauge, 6.0-cm needle connected to a 5-mL syringe containing a mixture of 4 mL of 0.5% lidocaine and 1 mL of 40 mg of triamcinolone was inserted into the brachialis at the MTrPs region. With the use of an in-plane method, the needle passing through the skin and adipose tissue to penetrate the muscle was visualized (Fig. 4). A physiatrist observed LTRs on US while performing trigger point injections. Repeated needling was performed to different loci in that region to elicit as many LTRs as possible. If no LTR was observed after 8 to 10 attempts, needling was stopped. At that point, mixture solution was injected. The injection site was pressed to ensure proper hemostasis after the procedure. No other therapy was allowed in each group during the study period (2 weeks for oral NSAID or 2 weeks after injection). However, self-exercise and behavior corrections were allowed. The patients were taught to do self-exercise comprising stretching exercise (repeated 10-20 times during a day) and to avoid the posture that might aggravate the symptoms.
Patients rated pain intensity using a 10-cm horizontal VAS, which varied from 'no pain (VAS 0)' to 'worst imaginable pain (VAS 10)'. VAS was assessed just before (pretreatment) and 2 weeks after the treatment (either trigger point injection or oral NSAID). 'Success of treatment' was defined as more than 50% of reduction in post-treatment VAS compared to pre-treatment, whereas 'treatment failure' was defined as <50% of post-treatment VAS reduction compared to pre-treatment. We compared the number of patients with successful treatments between each group.
Shapiro-Wilk test was used for all continuous variables for determining whether or not the distribution was normal. The general characteristics or baseline data were compared between each group by using Mann-Whitney U test or chi-square test. SPSS ver. 21.0 software (IBM, Armonk, NY, USA) was used for the statistical analyses. Statistical significance was considered when p-value was less than 0.05.
Of 72 patients who were diagnosed with rotator cuff disease, 29 developed upper arm pain during the follow-up period, 24 were unresponsive to subacromial injections, and two were excluded for having clinical impression of MPS of muscles other than brachialis, one had clinical suspicion for MPS on triceps brachii, one had on both triceps brachii and brachialis muscles. Twenty-two patients met the final inclusion criteria, and 21 patients completed the study. Eleven patients were allocated into the injection group, and 10 patients were in the oral NSAID group (Fig. 1).
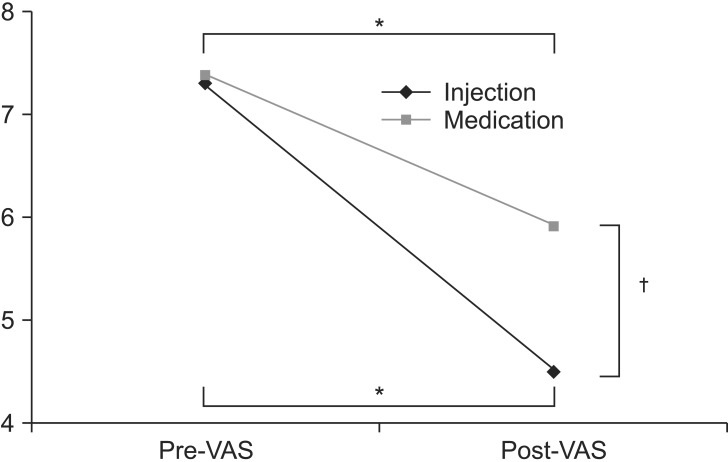
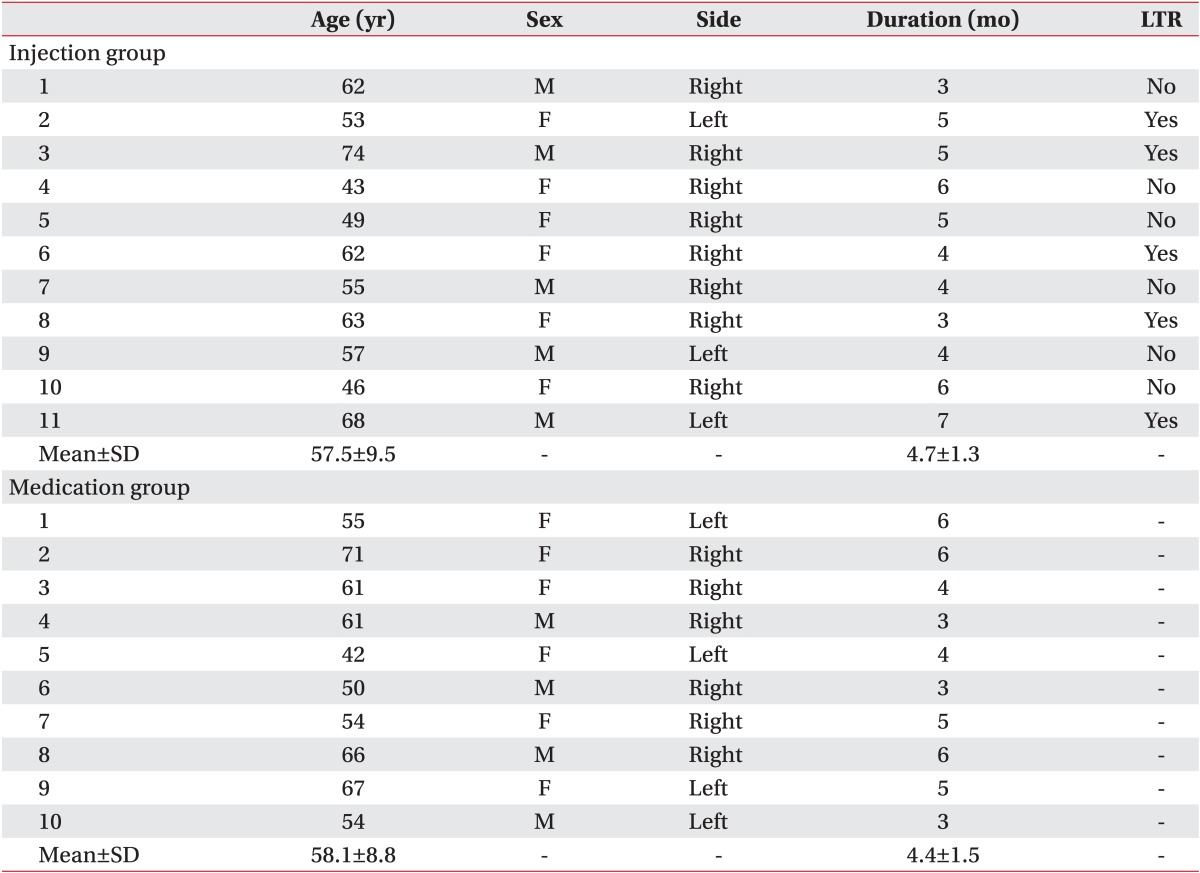
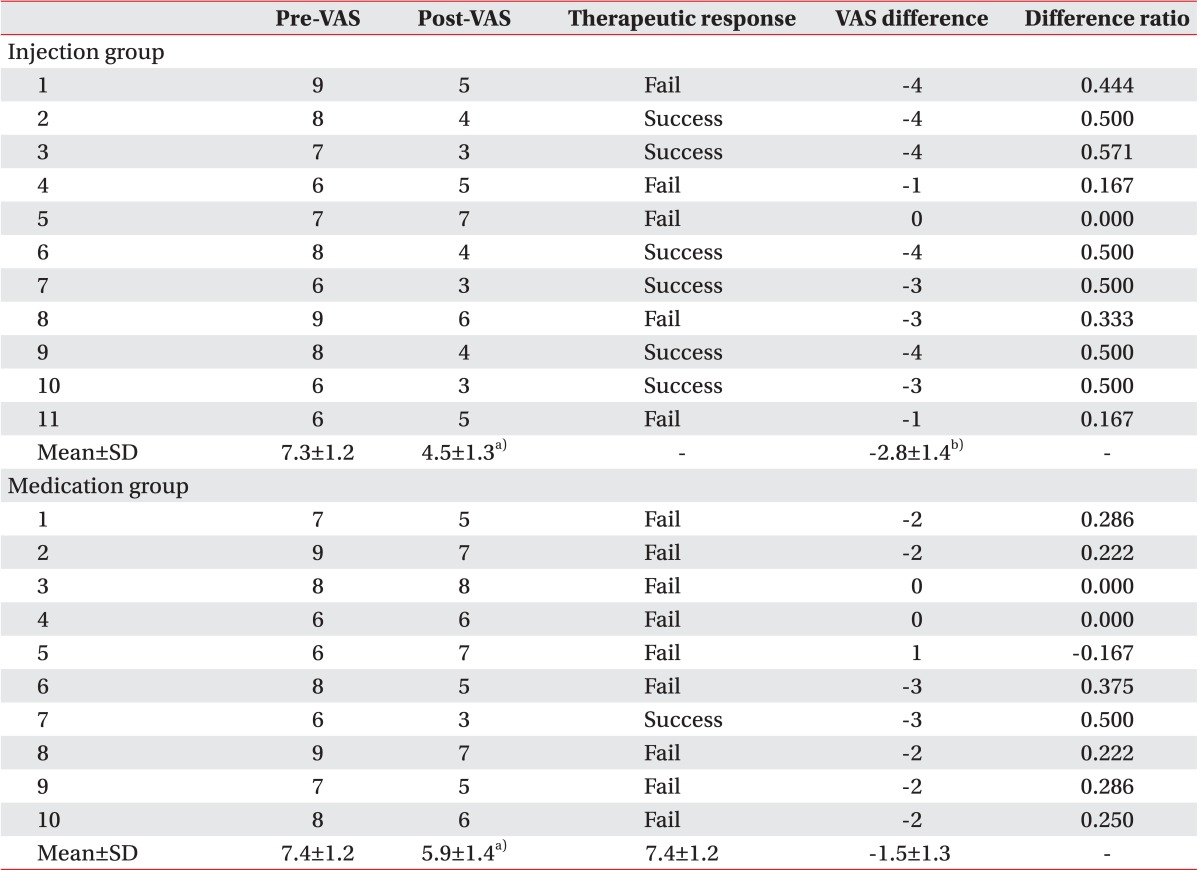
The mean age of patients included in the study was 57.5±9.5 years for the injection group and 58.1±8.8 years for the oral NSAID group. LTRs were observed during US-guided trigger point injection in 5 of 11 patients in the injection group. However, LTRs was not observed in the oral NSAID group who did not receive US-guided injection. Demographic and clinical characteristics of patients and assessment of treatments are summarized in Table 1. There was no significant difference in age, sex, or duration of symptoms between the two groups. No significant difference was observed in baseline VAS either between the two groups (7.3 in injection group vs. 7.4 in the oral NSAID group) (Table 2, Fig. 5). After 2 weeks, significant decreases of VAS after the treatment compared to the baseline VAS were observed in both groups (7.3 to 4.5 in the injection group and 7.4 to 5.9 in the oral NSAID group, p<0.05) (Table 2, Fig. 5). In addition, injection group had a significantly larger decrease of VAS score after the treatment compared to the oral NASID group (-2.8 in injection group vs. -1.5 in oral NSAID group, p<0.05) (Table 2, Fig. 5). The number of patients with 'successful treatment', whose post-treatment VAS reduced more than 50% compared to pre-treatment, was 6 of 11 (54.5%) in the injection group, whereas only one of 10 (10.0%) in the oral NSAID group had 'successful treatment'
In the injection group, no patient reported any complication related to the procedure or serious adverse events attributable to the treatment. There were no infections or vascular injuries. In the oral NSAID group, two patients had gastric pain or discomfort related to the treatment, but the symptoms subsided after ceasing the medication at the end of the 2-week follow-up period.
This is the first report of US-guided trigger point injections of the brachialis muscle in patients with rotator cuff disease. Affected upper arm pain significantly decreased in the injection group compared to the oral NSAID group. Rates of treatment success were 54.5% (6 of 11) in the injection group and 10.0% (1 of 10) in the oral NSAID group.
We only included those patients who were unresponsive to subacromial injection but with severe pain (VAS>5). Thus, even though the follow-up period was short (2 weeks), it could be concluded that US-guided trigger point injection of brachialis was effective in reducing pain of rotator cuff disease combined with pain originating from the brachialis muscle. Moreover, we used the US-guided method to improve efficacy and accuracy of injection. By palpating the brachialis before injection and precisely injecting the target muscle by US-guided method, the chance of misdirecting to a wrong muscle was very small.
No previous studies have reported on pain originating from the brachialis muscle combined with rotator cuff diseases. However, in our study, 29 out of 72 patients with rotator cuff disease had newly developed upper arm pain during the follow-up period. Twenty-four patients had upper arm pain unresponsive to subacromial injection, implying that the pain was different from a referred pain of the primary lesion, the rotator cuff tendon. All 24 patients were clinically suspected of MPS. Among them, 23 patients had MPS in the brachialis muscle, including one patient who also had MPS in the triceps brachii muscle. Our results suggest that the brachialis muscle is also important in the evaluation of patients with rotator cuff diseases.
MTrPs often result from, or are perpetuated by, acute or chronic overuse of the muscle or prolonged shortening of the muscle [11]. However, no previous studies have addressed the reason why patients with rotator cuff disease are having MPS of brachialis more frequently than having MPS of other muscles. Moreover, MTrPs in the brachialis are often incorrectly assessed as bicipital tendinitis, supraspinatus tendinitis, and C5 or C6 nerve compression [14].
More frequent dysfunction of the brachialis muscle than the biceps brachii muscle in patients with rotator cuff diseases could be explained by different characteristics of the two muscles including biomechanics. Firstly, the biceps brachii and brachialis are the two main elbow flexors. Which of these muscles plays a more important role in elbow flexion between biceps brachii and brachialis depends on the degree of elbow flexion and position changes. The brachialis, not involved in forearm supination, accounts for the larger portion in the elbow flexion moment during forearm pronation, whereas the biceps brachii is involved more in forearm supination [14]. Consequently, elbow flexion can be depicted as a combined force of the biceps and brachialis muscles in different situations. The biceps brachii, also a dynamic shoulder stabilizer, may play a relatively smaller role in elbow flexion in case of rotator cuff lesion. Overloading pressures of elbow flexion might have directed at the brachialis muscle instead [15,16]. Secondly, while the elbow is flexed, the main role of elbow flexion in the brachialis muscle is performed by the superficial head [17]. The proximal part of brachialis superficial head is attached to the humerus by partially encircling the distal tendon of the deltoid muscle [17,18], of which tendon and fibrous aponeurosis are continuous with the lateral intermuscular septum posteriorly and with the lateral aspect of the brachialis and deep brachial fascia anteriorly [18]. This tension may transfer to the brachialis muscle surrounding its distal end [19]. Thus, the repetitive tensions given to these structures may lead to MTrPs formation [11] in the brachialis muscle than in other muscles. Lastly, twojoint muscles including the biceps brachii that cross two or more joints are located more superficially, exhibiting faster contraction speeds with relatively reduced force production [20]. Unlike the two-joint muscles, one-joint muscles like the brachialis have a slower contraction speed with increased force production. Those muscles that cross one joint are usually located closer to bone and generally more involved in postural activities. Distorted posture due to shoulder pain may augment tissue changes in the brachialis muscle, therefore adversely affecting the brachialis. Thus, position change in scapula and shoulder due to the rotator cuff disease may have loaded more pressure on the brachialis muscle. Moreover, shoulder pain induced by reasons other than rotator cuff disease or long-term fixation in elbow flexion position may also induce brachialis MPS.
Since not all patients showed LTRs during the injection procedure, the source of the pain from the brachialis muscle might not solely be MPS. Chronic muscle problems usually are caused by a combination of stretchinduced muscle injury, muscle strain, and muscle spasm along with MPS. Therapeutic injection of steroids and anesthetics under US-guidance reportedly sped recovery and rehabilitation in professional baseball pitchers with internal oblique muscle strain [21]. Combined therapy with steroid and local anesthetic was effective for pain relief in three women who had adductor muscle strain which was not improved 3 months after transobturator taping [22]. Another study showed that intramuscular injection of steroid was effective in patients suffering from chronic myofascial pain or pain from chronic muscle spasm in the piriformis, iliopsoas or scalenus anterior muscles, whereas the effect had begun to wane at 30 days post-injection [23]. However, follow-up period of our study was only 2 weeks. Therefore, further studies on long-term effect are needed. Even if it was muscle strain, not MPS, that induced the pain, brachialis muscle overloading may induce dysfunction of brachialis muscle for the same reason. Considering the results of this study, pain originating in the musculature of the shoulder girdle or upper arm should also be evaluated in case of rotator cuff disease. Patients with severe pain originating from brachialis muscle who do not respond well to oral NSAIDs as shown in our study should be treated with alternatives including intramuscular injection.
Limitations of our study include the small number of participants and the short-term period of follow-up. Not having any previous studies concerning the brachialis-originated pain, we conducted a pilot study with a small number of patients to prove its efficacy first. The long-term effect of intramuscular steroid injection of the brachialis for patients with rotator cuff disease remains to be determined. There was no evidence that intramuscular steroid injection is the choice of treatment for the muscle strain or MPS. However, the purpose of this study was to investigate whether the brachialis was the cause of upper arm pain in patients with rotator cuff disease, not to determine the optimal treatment. The best treatment strategy may not be the trigger point injections with local anesthetics and steroid for the brachialis-originated pain, which needs to be studied in the future.
According to our single-blind, randomized, and controlled pilot study, US-guided trigger point injection of the brachialis provided more significant pain relief in patients with rotator cuff disease when compared to oral NSAID medications. These findings suggest that US-guided trigger point injections of the brachialis could be a safe and useful treatment for upper arm pain in patients with rotator cuff disease. Among the patients with rotator cuff disease, the presence of combined brachialis muscle-originated severe pain may extend the length of overall treatment period and result in worse outcome. Thus, further study on the brachialis-originated pain in rotator cuff disease, focusing on its frequency, etiology, natural course, and optimal treatment, will be necessary.
References
1. Toivonen DA, Tuite MJ, Orwin JF. Acromial structure and tears of the rotator cuff. J Shoulder Elbow Surg. 1995; 4:376–383. PMID: 8548441.

2. Andrews JR. Diagnosis and treatment of chronic painful shoulder: review of nonsurgical interventions. Arthroscopy. 2005; 21:333–347. PMID: 15756189.

3. Chou WY, Ko JY, Wang FS, Huang CC, Wong T, Wang CJ, et al. Effect of sodium hyaluronate treatment on rotator cuff lesions without complete tears: a randomized, double-blind, placebo-controlled study. J Shoulder Elbow Surg. 2010; 19:557–563. PMID: 19963403.

4. Hong CZ. Pathophysiology of myofascial trigger point. J Formos Med Assoc. 1996; 95:93–104. PMID: 9064014.
5. Hong CZ, Simons DG. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch Phys Med Rehabil. 1998; 79:863–872. PMID: 9685106.

7. Bron C, Wensing M, Franssen JL, Oostendorp RA. Treatment of myofascial trigger points in common shoulder disorders by physical therapy: a randomized controlled trial (ISRCTN75722066). BMC Musculoskelet Disord. 2007; 8:107. PMID: 17983467.

8. Bron C, Dommerholt J, Stegenga B, Wensing M, Oostendorp RA. High prevalence of shoulder girdle muscles with myofascial trigger points in patients with shoulder pain. BMC Musculoskelet Disord. 2011; 12:139. PMID: 21711512.

9. Calais-Germain B. The elbow. Anatomy of movement. Seatle: Eastland Press;1993. p. 131–146.
10. Hong CZ. Specific sequential myofascial trigger point therapy in the treatment of a patient with myofascial pain syndrome associated with reflex sympathetic dystrophy. Australas Chiropr Osteopathy. 2000; 9:7–11. PMID: 17987165.
11. Travell JG, Simons DG. Myofascial pain and dysfunction: the trigger point manual. 2nd ed. Baltimore: Williams & Wilkins;1999.
12. Rha DW, Shin JC, Kim YK, Jung JH, Kim YU, Lee SC. Detecting local twitch responses of myofascial trigger points in the lower-back muscles using ultrasonography. Arch Phys Med Rehabil. 2011; 92:1576–1580.e1. PMID: 21839982.

13. Kim DS, Jeong TY, Kim YK, Chang WH, Yoon JG, Lee SC. Usefulness of a myofascial trigger point injection for groin pain in patients with chronic prostatitis/ chronic pelvic pain syndrome: a pilot study. Arch Phys Med Rehabil. 2013; 94:930–936. PMID: 23262156.
14. Muscolino JE. The muscle and bone palpation manual with trigger points, referral patterns, and stretching. 1st ed. St. Louis: Mosby/Elsevier;2009.
15. Landin D, Myers J, Thompson M, Castle R, Porter J. The role of the biceps brachii in shoulder elevation. J Electromyogr Kinesiol. 2008; 18:270–275. PMID: 17196396.

16. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, O'Brien SJ. Role of the long head of the biceps brachii in glenohumeral stability: a biomechanical study in cadavera. J Shoulder Elbow Surg. 1996; 5:255–262. PMID: 8872922.

17. Leonello DT, Galley IJ, Bain GI, Carter CD. Brachialis muscle anatomy: a study in cadavers. J Bone Joint Surg Am. 2007; 89:1293–1297. PMID: 17545433.
18. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009; 18:386–390. PMID: 19186076.

19. McCully SP, Suprak DN, Kosek P, Karduna AR. Suprascapular nerve block results in a compensatory increase in deltoid muscle activity. J Biomech. 2007; 40:1839–1846. PMID: 17034796.

20. DeLisa JA, Gans BM, Bockenek WL, Frontera WR, Gerber LH, Geiringer SR, et al. Physical medicine and rehabilitation: principles and practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins;2005.
21. Stevens KJ, Crain JM, Akizuki KH, Beaulieu CF. Imaging and ultrasound-guided steroid injection of internal oblique muscle strains in baseball pitchers. Am J Sports Med. 2010; 38:581–585. PMID: 20051499.

22. Roth TM. Management of persistent groin pain after transobturator slings. Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:1371–1373. PMID: 17431534.

23. Porta M. A comparative trial of botulinum toxin type A and methylprednisolone for the treatment of myofascial pain syndrome and pain from chronic muscle spasm. Pain. 2000; 85:101–105. PMID: 10692608.

Fig. 2
Proper positioning during injection procedure. (A) The patient is laid on the lateral side with the affected side up, with the upper arm placed tightly to the trunk to minimize the motion during injection. (B) The probe is positioned on the maximal tender point to show the transverse image of the brachialis muscle, and the needle is approached with inplane method.
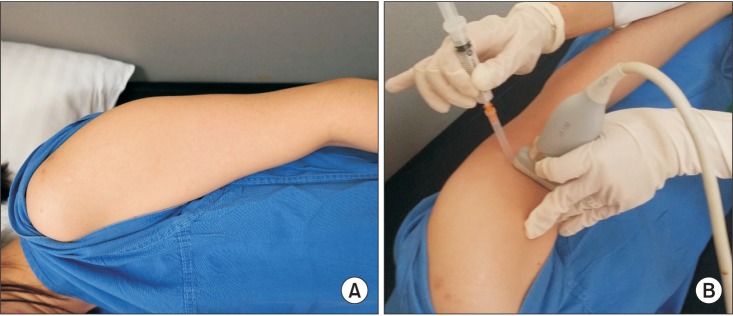
Fig. 3
Transverse ultrasound image of the brachialis muscle. The brachialis muscle (Br) is seen below the biceps brachii muscle (Bi). H, humerus; T, triceps.
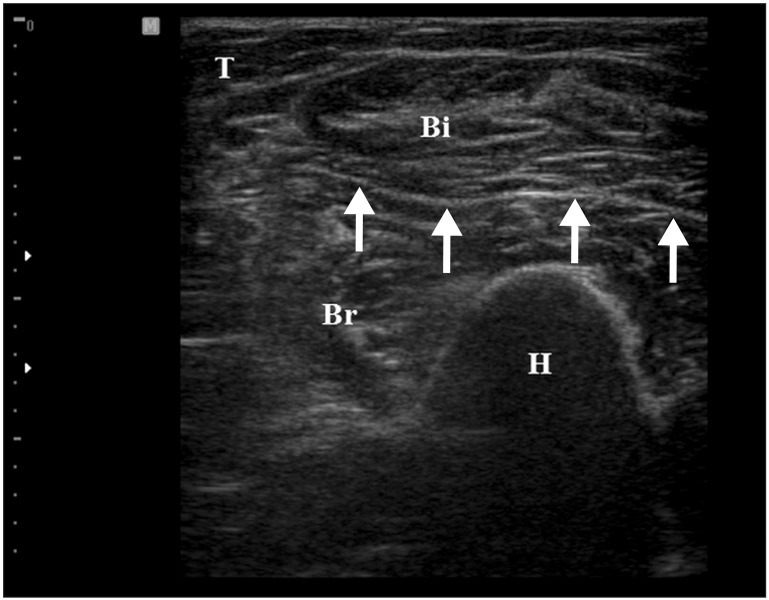
Fig. 4
Transverse ultrasound image by the in-plane method. Needle passage into the target muscle (Br) is visualized (arrow). Bi, biceps brachii; Br, brachialis; H, humerus.
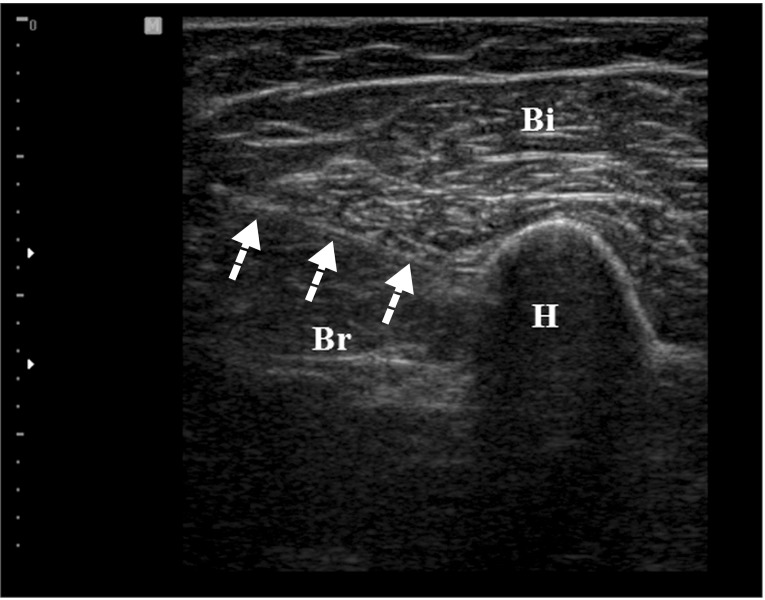
Fig. 5
Effect of each treatment. VAS changes significantly after injection and medication (*p<0.05) and VAS in injection group decreases more compared to the medication group after treatment (†p<0.05). VAS, visual analogue scale; pre-VAS, VAS at pretreatment; post-VAS, VAS after 2 weeks of treatment.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download