Abstract
Objective
To delineate whether cortical plasticity induced by continuous theta burst stimulation (cTBS) differed according to catechol-O-methyltransferase (COMT) gene polymorphism in healthy older adults.
Methods
Eighteen healthy older volunteers (mean age 73.78±5.04; 12 females and 6 males) were recruited. Volunteers randomly assigned in either a sham-first or real cTBS first group participated in two separate TMS visits with at least a 2-day wash-out period. Genotyping was carried out at baseline by a separate researcher who was blinded. cTBS was delivered in a hot spot over M1 at an active motor threshold of 80%. Motor evoked potentials (MEPs) were obtained at 120% of the resting motor threshold before and after sham/cTBS.
Results
The relative MEP to baseline was significantly decreased 0 and 10 minutes post-stimulation and increased 40 minutes post-stimulation, as compared with the sham condition. Immediately after cTBS, the Val/Val group had a significantly reduced relative MEP value, as compared with the MET carrier group.
Transcranial magnetic stimulation (TMS) has become a valuable tool to non-invasively investigate plasticity in the human brain [1,2]. Repetitive TMS (rTMS) can induce brain plasticity that lasts from several minutes to an hour with a focally stimulated cortical area, as well as the related cortical and subcortical areas [3]. A specific patterned rTMS protocol, known as theta burst stimulation (TBS) [4], induces particularly robust physiological after-effects depending on the continuous or intermittent pattern, leading to either decreased or increased excitability in healthy subjects, despite being short and relatively low in intensity [4,5]. These protocols have been shown to be useful to assess changes in brain plasticity mechanisms related to normal aging [6], as well as in pathologic conditions [7]. However, even in healthy subjects, responses to these protocols are highly variable among different individuals. A number of factors have already been described that contribute to this variation, such as age [8] and menstrual cycle [9]. Genetic factors might also influence TBS-induced plasticity [10,11,12].
Catechol-O-methyltransferase (COMT) is a plasticity related gene that influences the availability of dopamine in the synaptic cleft by stimulating its degradation [7]. The substitution of methionine (Met) for valine (Val) at codon 158 on chromosome 22q11 in the COMT gene [13] is associated with faster dopamine degradation and a lower dopamine concentration in the synaptic cleft resulting in better executive function and memory performance in COMT Met carriers [14,15,16]. Also, dopaminergic signaling in the primary motor cortex (M1) is necessary for normal motor skill learning and synaptic plasticity within M1 [17].
Although many possible factors might influence the continuous theta burst stimulation (cTBS)-induced plasticity in the motor cortex, only one study has indirectly showed a relationship of paired associative stimulation (PAS)-related plasticity regarding COMT polymorphism [18].
This study investigated the effect of COMT polymorphism on cTBS-induced motor plasticity in healthy older persons based on the hypothesis that the Met carrier group would have greater plasticity than the Val/Val group.
Eighteen right-handed older volunteers (mean±standard deviation age, 73.78±5.04 years) were recruited using the following inclusion criteria: 1) no history of neurological or psychiatric disorders, 2) no drug abuse or use of central nervous system medication, and 3) normal neurological examination with Mini-Mental State Examination (MMSE) scores in the normal range (27-30). Volunteers who had lesions including small vessel disease with magnetic resonance imaging were excluded. All participants gave written informed consent for the study that followed international guidelines and recommendations for the safe use of TMS [19], and the ethical committee of Hallym University Sacred Heart Hospital approved the study.
Participants were informed, had two separate TMS visits with at least a 2-day wash-out period, and had genotyping performed at the baseline visit. Subjects were divided first into either a sham or real cTBS group by random assignment (Fig. 1).
At the baseline visit, all participants underwent a venous blood sample for genotype analysis. DNA extraction was performed from ethylenediaminetetraacetic acid-blood samples of all probands according to standard protocols. Ethical approval for genotyping was provided by the ethics committee of the Hallym University. Genomic DNA extracted from peripheral lymphoblasts was used for sequencing. To examine polymorphisms of the COMT gene (rs4680), polymerase chain reaction (PCR) and Sanger sequencing of exon 4 in COMT were done. Briefly, the reaction mixture contained 1 µL of gDNA of 50 ng, 3 µL of 10× PCR buffer, 3 µL of 2.5 mM dNTP, 1 µL of forward primer (5'-GGGCCTACTGTGGCTA CTCA-3'), 1 µL of reverse primer (5'-GTGGTCGAGGAAGACCATGT-3'), and 0.2 µL of Taq polymerase that was added to water (total volume, 30 µL). All samples were amplified at 94℃ for 30 seconds, 55℃ for 30 seconds, and 72℃ for 45 seconds for 30 cycles and sequenced using an ABI 3730 sequencer. Based on their Val-Met allele carrier status, participants were classified into two genotype groups: homozygous Val/Val carriers (n=9) versus one or two Met-allele carriers (n=9).
Different investigators sampled blood and performed a genotyping analysis separately from the TMS study to maintain blinding.
The stimulation set-up consisted of a Magnetic Stimulator STM 9000 (ATES MEDICA Device, Italy) for single-pulse TMS and cTBS intervention. A figure-8 coil was placed tangentially over the left primary motor cortex, with the handle pointing at a 45° angle posterolaterally. For the MEP measurement, surface electromyography (EMG) was recorded using pre-gelled, disposable Ag/AgCl electrodes with the active electrode in the contralateral first dorsal interosseous (FDI) muscle, the reference electrode over the metacarpophalangeal joint, and the ground electrode over the wrist. The EMG signal was acquired at 3 kHz, filtered (10-500 Hz), amplified, and stored for offline analysis.
The participants were seated in a comfortable chair with a headrest and had their hands resting on their laps. They were monitored for drowsiness and asked to keep their eyes open during TMS. All participants wore earplugs to protect them from possible acoustic trauma and to reduce noise from the discharge of the TMS coil.
The resting motor threshold (RMT) was obtained over M1, where the lowest stimulus intensity evoked TMS-induced motor evoked potentials (MEPs) of at least 50 µV in five out of 10 consecutive trials in the target muscle. The active motor threshold (AMT) was defined as the lowest TMS intensity capable of inducing visible FDI twitches in half of the trials, while the participants maintained an FDI contraction of approximately 20% of the maximal voluntary contraction [20]. The cTBS protocol consisted of 600 pulses at an AMT intensity of 80%, delivered in trains of three pulses at 50 Hz and repeated every 200 ms for a total of 40 seconds [4]. Sham stimulation was conducted with the same stimulus intensity and duration, with the coil tilted 90° during stimulation.
To establish a pre-cTBS baseline measure, two batches of 10 MEPs were recorded in response to a single TMS pulse at an RMT intensity of 120%. The pulses were delivered with interstimulus intervals of at least 5 seconds. Following cTBS, a single batch of MEPs was measured immediately thereafter (T0) and then at 5, 10, 20, 30, 40, and 60 minutes (Fig. 1).
The peak-to-peak amplitude of each MEP was determined trial-by-trial through visual inspection. For each subject, the baseline pre-cTBS MEP amplitude was defined as the average peak-to-peak amplitude of the MEPs recorded during two pre-cTBS batches. The MEP amplitude at the time (T) after cTBS was defined as the average peak-to-peak amplitude for the MEPs recorded during the corresponding batch; this value was then expressed as a change in MEPs, as compared with the pre-cTBS condition, i.e., [MEPs (T) - MEPs (pre-cTBS)] / MEPs (pre-cTBS). The time to baseline was defined as the time point at which the post-cTBS MEP amplitude returned to the MEP amplitude at baseline.
First, for estimating the group effect between the sham and real cTBS conditions over time, as compared with baseline, repeated measures ANOVA was performed using stimulation mode (real cTBS versus the sham condition) as a between-subject factor and time (baseline, T0, T10, T20, T30, T40, and T60) as a within-subject factor. Multiple comparison correction was done using Fisher least significant difference (LSD) method. Second, for estimation of the COMT polymorphism effect between Val/Val and Met carriers, repeated measures ANOVA with genotype (Val/Val, Met carriers) as a within-subject factor and time (baseline, T0, T10, T20, T30, T40, and T60) as a within-subject factor and between-subject factor. A multiple comparison correction was done using the Bonferroni method. Third, a Spearman correlation analysis was conducted for other factors including age, MMSE, gender, RMT, AMT, and time to baseline.
For repeated measure ANOVA, the sphericity assumption was first checked with Mauchly test, and Huynh-Feldt corrections were applied if the assumption of sphericity was violated (ε<0.7); the degrees of freedom were adjusted for the averaged results from the significance test using the F test whenever sphericity was not assumed. Normal distribution of the data was assessed with the Kolmogorov-Smirnov and Shapiro-Wilk tests, which was in accordance with a normal distribution between the MEPs of a Val/Val homozygote and Met allele carriers. Statistical analyses were done using SPSS ver. 18.0 (SPSS Inc., Chicago, USA), with a p<0.05 indicating significance.
The participants' demographics are shown in Table 1. Regarding the COMT genotype, 9 participants had the Val/Val allele, while 5 participants were Val/Met carriers, and 4 participants were Met/Met allele carriers. No significant differences were noted between groups. No subjects experienced any TMS side effects.
Repeated measures ANOVA revealed no significant effect in stimulation mode, while a significant effect was observed with the time factor (F=4.969; p=0.001), along with a significant interaction between time and stimulation mode (F=4.014; p=0.003). A post-hoc LSD test was significant between the baseline MEP and T0 (p=0.006), T10 (p=0.015), and T40 (p=0.006) (Fig. 2). After returning to baseline, the MEP significantly increased at T40 post-stimulation, which returned to baseline at T60.
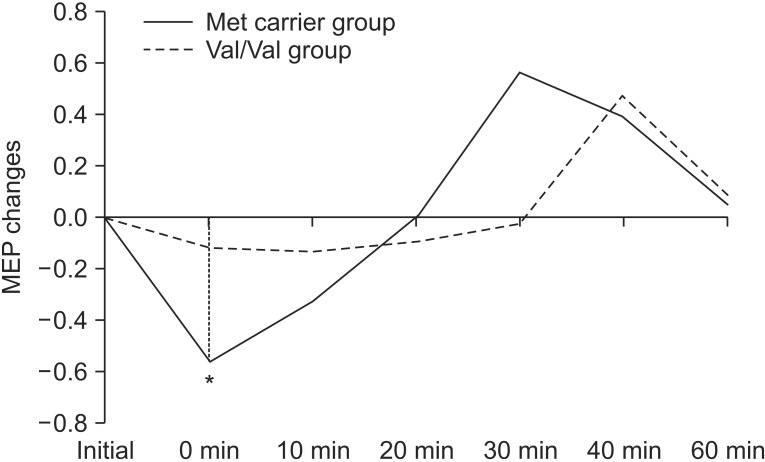
Repeated measures ANOVA showed no significant effect with genotype, while a significant effect was observed with time (F=7.159; p<0.001), along with a significant interaction between time and genotype (F=2.433; p=0.046) (Fig. 3). There was a significant difference with the post-hoc test using Bonferroni method between baseline and T0 (p<0.001).
The correlation between the time to return to baseline after stimulation and other factors (age, gender, MMSE, RMT, AMT, and initial MEP) was not statistically significant.
The duration of the cTBS effect, which was measured by the time to baseline, lasted 25.56±17.4 seconds for the Val/Val group and 28.89±16.16 seconds for the Met carrier group. There was no statistically significant relationship between the two groups.
cTBS intervention induced suppression in healthy older
subjects, as expected. The effect of COMT gene polymorphism on cTBS-induced motor plasticity in healthy older adults was different between the Val/Val group and Met carrier group, and was significantly less in the former. To the best of our knowledge, this study is the first showing a COMT polymorphism effect with cTBS.
COMT gene polymorphism-dependent differences in dopaminergic transmission have an important behavioral relevance related to executive function [14,15,16] and motor learning [21], as dopamine affects long-term potentiation (LTP) and neuronal plasticity [22,23]. Basically, the COMT gene is crucially implicated in central dopamine function as the enzyme that was derived from the COMT gene degrades dopamine in the synapse. Functional COMT polymorphisms in Met carriers are expected to have decreased enzyme activity leading to a reduction in dopamine catabolism in the frontal cortex [24]. A nonlinear, inverted U-shaped dose-response curve for the effects of dopamine on cognition has been observed [24,25]. The application of both the D1-receptor and D2-receptor antagonists markedly reduced the ability of M1 horizontal connections to form an LTP in an animal study [17], suggesting the necessity of dopamine signaling for long-lasting synaptic plasticity in M1, which has been shown using TMS [26].
In this study, we found differences in cTBS-induced motor cortex plasticity between Val158Val and Met carriers. In particular, the immediate response (T0) was decreased in Val158Val compared with the Met carriers. A previous study using PAS suggested having immediate LTP-like plasticity showing a genetic interaction between brain-derived growth factor (BDNF) and COMT [18], which is compatible with our results, although they used different neuromodulatory technique. The latter authors showed that the BDNF Val/Val genotype in combination with the COMT Met/Met genotype group had an increased LTP and that the group had higher immediate learning success with a grammar task, although the group did not experience statistical significance with a motor learning task. Moreover, directly after PAS, the level of LTP induction correlated well with motor learning performance. Considering all these points and prior results [27] in another plasticity related gene, it is reasonable to expect similar plasticity with cTBS.
Of note, the average age in this study was about 74 years. The dopamine system undergoes a marked decline with increasing adult age, with gradual loss of both pre- and post-synaptic markers in dopamine neurotransmission [28,29,30,31], and with age-related impairment of multiple cognitive tasks including those assessing working memory and executive functions [29,32,33]. Freitas et al. [6] reported that the duration of the cTBS effect correlates linearly and inversely with age. Additionally, in our study, the duration of the cTBS effect measured by the time to baseline lasted only 25.56 seconds for the Val/Val group and 28.90 seconds for the Met carrier group. Given the close association between deficient dopaminergic neuromodulation, it is plausible to assume that increasing adult age shifts individuals away from the functional optimum that is particularly pronounced among individuals with Val carriers of the COMT gene who have relatively low dopamine levels. In our opinion, one of the main reasons why our study showed distinct differences in plasticity according to COMT polymorphism is because of the relatively older age groups recruited for this study; this was not the case in most of the previous studies [14,18,21,34].
There was a significant increase in MEP 40 minutes post-stimulation in the cTBS group, as compared with the sham condition. This is thought to be a variable after effect of cTBS because of individual differences in the recruitment of cortical neurons [35]. Individual genotypes could influence neuroplasticity induction, as well as age [8] and menstrual cycle [9]. In addition, our findings could be interpreted in the context of homeostatic plasticity for the human motor cortex, keeping the plasticity threshold within a dynamic range to prevent extreme changes in synaptic efficacy [36]. Further study is warranted to learn more about these factors [37].
There were some limitations in this study. First, the COMT genotype was expected with the Hardy-Weinberg equilibrium (p>0.05). But, because of the small sample size, the COMT polymorphism distributions were not as expected so we could not subgroup the genotype into Val/Val, Val/Met, and Met/Met group, or the Val/Val and Met/Met group. Study of a larger population is warranted. Secondly, we did not measure executive function and learning ability in this study, which would have greatly strengthened the results.
We found that cTBS intervention induced suppression of MEPs in healthy older adults, as expected. The effect of COMT gene polymorphism on cTBS-induced motor plasticity in healthy older adults differed between the Val/Val group and Met carrier group. To the best of our knowledge, this study is the first showing the COMT polymorphism effect on cTBS.
ACKNOWLEDGMENTS
This research was supported by the Korea Research Foundation grant funded by the Korean government (MOEHRD, Basic Research Promotion Fund; No. 521-2008-1-E00099), the National Research Foundation of the Korea grant funded by the Korean government (MEST, Basic Research Promotion Fund; No. NRF-2013R1A1A2012562), and Hallym University Medical Center Research Fund (No. 01-2012-06).
References
1. Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002; 543(Pt 2):699–708. PMID: 12205201.

2. Pascual-Leone A. Disrupting the brain to guide plasticity and improve behavior. Prog Brain Res. 2006; 157:315–329. PMID: 17167918.

3. Lee L, Siebner H, Bestmann S. Rapid modulation of distributed brain activity by Transcranial Magnetic Stimulation of human motor cortex. Behav Neurol. 2006; 17:135–148. PMID: 17148833.

4. Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005; 45:201–206. PMID: 15664172.

5. Doeltgen SH, Ridding MC. Low-intensity, short-interval theta burst stimulation modulates excitatory but not inhibitory motor networks. Clin Neurophysiol. 2011; 122:1411–1416. PMID: 21195662.

6. Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, et al. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011; 3:5. PMID: 21519394.

7. Oberman L, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, et al. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile x syndrome and autism spectrum disorder. Front Synaptic Neurosci. 2010; 2:26. PMID: 21423512.

8. Muller-Dahlhaus JF, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res. 2008; 187:467–475. PMID: 18320180.

9. Inghilleri M, Conte A, Curra A, Frasca V, Lorenzano C, Berardelli A. Ovarian hormones and cortical excitability: an rTMS study in humans. Clin Neurophysiol. 2004; 115:1063–1068. PMID: 15066531.

10. Lee M, Kim SE, Kim WS, Lee J, Yoo HK, Park KD, et al. Interaction of motor training and intermittent theta burst stimulation in modulating motor cortical plasticity: influence of BDNF Val66Met polymorphism. PLoS One. 2013; 8:e57690. PMID: 23451258.

11. Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010; 3:230–237. PMID: 20965453.

12. Li Voti P, Conte A, Suppa A, Iezzi E, Bologna M, Aniello MS, et al. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp Brain Res. 2011; 212:91–99. PMID: 21537966.

13. Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004; 8:325–335. PMID: 15242692.

14. Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val-158met genotype. Biol Psychiatry. 2006; 60:1250–1258. PMID: 16950222.

15. de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet. 2004; 34:533–539. PMID: 15319576.

16. Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009; 23:105–116. PMID: 19210038.

17. Molina-Luna K, Pekanovic A, Rohrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti MS, et al. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One. 2009; 4:e7082. PMID: 19759902.

18. Witte AV, Kurten J, Jansen S, Schirmacher A, Brand E, Sommer J, et al. Interaction of BDNF and COMT polymorphisms on paired-associative stimulation-induced cortical plasticity. J Neurosci. 2012; 32:4553–4561. PMID: 22457502.

19. Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009; 120:2008–2039. PMID: 19833552.

20. Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994; 91:79–92. PMID: 7519144.

21. Noohi F, Boyden NB, Kwak Y, Humfleet J, Burke DT, Muller ML, et al. Association of COMT val158met and DRD2 G>T genetic polymorphisms with individual differences in motor learning and performance in female young adults. J Neurophysiol. 2014; 111:628–640. PMID: 24225542.
22. Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann N Y Acad Sci. 2003; 1003:241–249. PMID: 14684450.

23. Li XH, Wang JY, Gao G, Chang JY, Woodward DJ, Luo F. High-frequency stimulation of the subthalamic nucleus restores neural and behavioral functions during reaction time task in a rat model of Parkinson's disease. J Neurosci Res. 2010; 88:1510–1521. PMID: 20025062.

24. Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Backman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci. 2008; 2:234–244. PMID: 19225597.
25. Li SC, Sikstrom S. Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neurosci Biobehav Rev. 2002; 26:795–808. PMID: 12470691.

26. Lang N, Speck S, Harms J, Rothkegel H, Paulus W, Sommer M. Dopaminergic potentiation of rTMS-induced motor cortex inhibition. Biol Psychiatry. 2008; 63:231–233. PMID: 17604004.

27. Cheeran BJ, Ritter C, Rothwell JC, Siebner HR. Mapping genetic influences on the corticospinal motor system in humans. Neuroscience. 2009; 164:156–163. PMID: 19409217.

28. Leenders KL, Antonini A, Thomann R, Locher JT, Maitre L, Gerebtzoff A, et al. Savoxepine: striatal dopamine-D2 receptor occupancy in human volunteers measured using positron emission tomography (PET). Eur J Clin Pharmacol. 1993; 44:135–140. PMID: 8095895.

29. Erixon-Lindroth N, Farde L, Wahlin TB, Sovago J, Halldin C, Backman L. The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res. 2005; 138:1–12. PMID: 15708296.

30. Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000; 21:683–688. PMID: 11016537.

31. Suhara T, Fukuda H, Inoue O, Itoh T, Suzuki K, Yamasaki T, et al. Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology (Berl). 1991; 103:41–45. PMID: 1826059.

32. Backman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C, et al. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000; 157:635–637. PMID: 10739428.
33. Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998; 155:344–349. PMID: 9501743.
34. Enoch MA, Waheed JF, Harris CR, Albaugh B, Goldman D. COMT Val158Met and cognition: main effects and interaction with educational attainment. Genes Brain Behav. 2009; 8:36–42. PMID: 19076243.

35. Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 2013; 23:1593–1605. PMID: 22661405.

36. Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000; 10:358–364. PMID: 10851171.

37. Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010; 588(Pt 13):2291–2304. PMID: 20478978.

Fig. 1
Timeline of an experimental session. MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; MEP, motor evoked potential; cTBS, continuous theta burst stimulation.
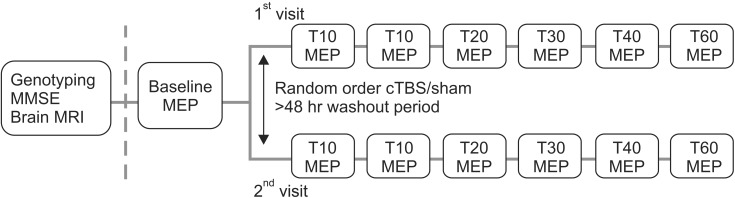
Fig. 2
Motor evoked potential (MEP) changes after continuous theta burst stimulation (cTBS) and sham stimulation (*p<0.05).
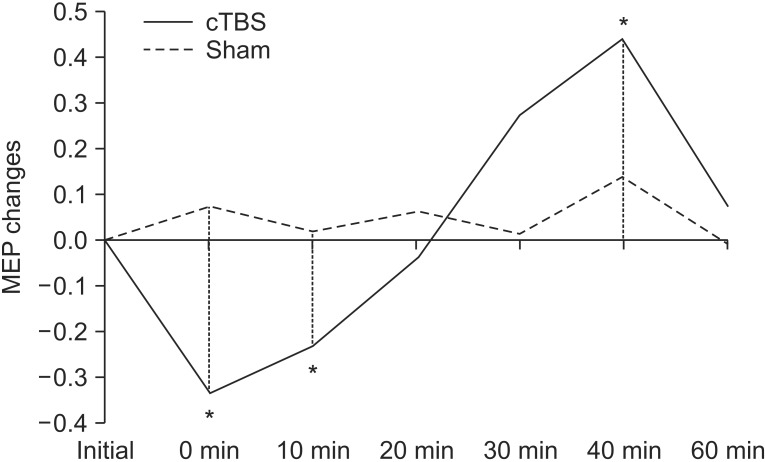
Fig. 3
Motor evoked potential (MEP) changes postcTBS between the Met carrier group and Val/Val group (*p<0.05). cTBS, continuous theta burst stimulation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download