CASE REPORT
A 57-year-old man with poorly controlled diabetes mellitus diagnosed 1 year ago (HbA1c 8.5% at admission) visited the local emergency room complaining of sudden left arm weakness. He denied any recent immunizations, preceding trauma (including procedures, such as acupuncture or apitoxin), or infection. A laboratory study and shoulder X-ray showed no evidence of abnormalities. Left shoulder pain and swelling developed after several hours and rapidly extended to the left hand. The patient visited another hospital and magnetic resonance imaging (MRI) revealed the formation of a subdeltoid abscess along with septic arthritis in the left shoulder joint (
Fig. 1). He was transferred to the department of orthopedic surgery of our hospital for surgery 9 days after the onset of symptoms. His vital signs at admission were stable without fever. The laboratory data showed abnormal values, including white blood cell count of 18,210/µL (normal range, 4,0000-10,000/µL), C-reactive protein of 31.42 mg/dL (normal range, 0-0.3 mg/dL), and erythrocyte sedimentation rate of 101 mm/hr (normal range, 0-15 mm/hr). A physical examination revealed painful limitation of motion, tenderness, swelling, and a heat sensation in the left shoulder area. Intravenous antibiotics (cefazolin and clindamycin) were started, and open irrigation and debridement were performed through arthrotomy on the next day.
S. agalactiae was identified by culture, and it was susceptible to most antibiotics; thus, cefazolin and clindamycin were changed to amoxicillin.
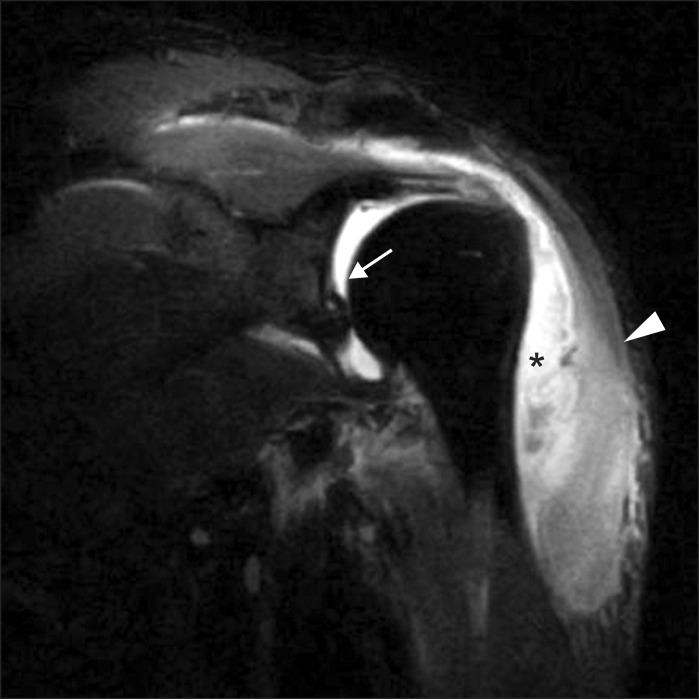 | Fig. 1Magnetic resonance imaging (T1) shows myositis of the deltoid muscle (arrowhead), subdeltoid abscess formation (asterisk), and septic arthritis in the left shoulder joint (arrow). 
|
The left upper extremity weakness and hypesthesia continued after surgery. An electrodiagnostic examination was performed about 4 weeks after the onset of initial symptoms. The manual muscle test revealed severe weakness of the left supraspinatus, infraspinatus, and deltoid muscles (Medical Research Council [MRC] grade, 1/5) with mild weakness of the left biceps and triceps muscles (MRC, 4/5). Wrist and hand power was almost normal. A sensory examination showed paresthesia in the left axillary nerve distribution. Moderate to severe atrophy of the left supraspinatus, infraspinatus, and deltoid muscles was noticed, and mild atrophy of the left biceps and triceps muscles was also found (
Fig. 2). Electromyography and nerve conduction studies (NCS) showed left upper and middle trunk brachial plexopathy but involving the upper trunk more severely. The axillary, musculocutaneous, and suprascapular nerves were absent of compound muscle action potentials (CMAPs) when recorded from the deltoid, biceps, and supraspinatus muscles, respectively (
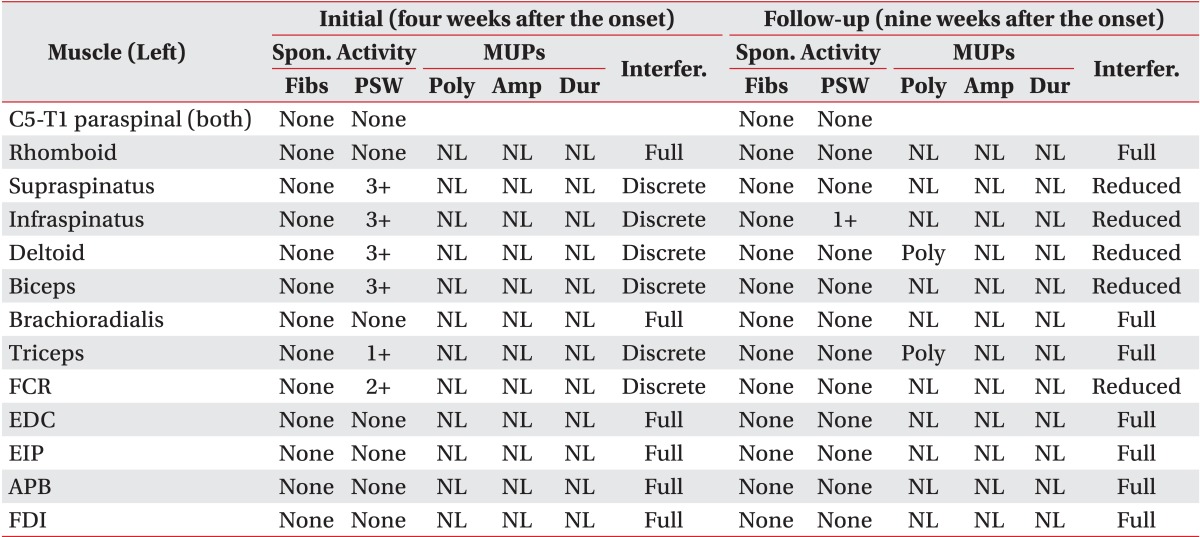
Table 1). The left supraspinatus, infraspinatus, deltoid, biceps, triceps, and flexor carpi radialis muscles showed discrete interference patterns and abnormal spontaneous activities on electromyography (
Table 2). Motor unit action potentials (MUAPs) of all sampled muscles were unremarkable.
 | Fig. 2Photograph shows atrophy of the left supraspinatus, infraspinatus, and deltoid muscles. 
|
Table 1
Results of nerve conduction studies
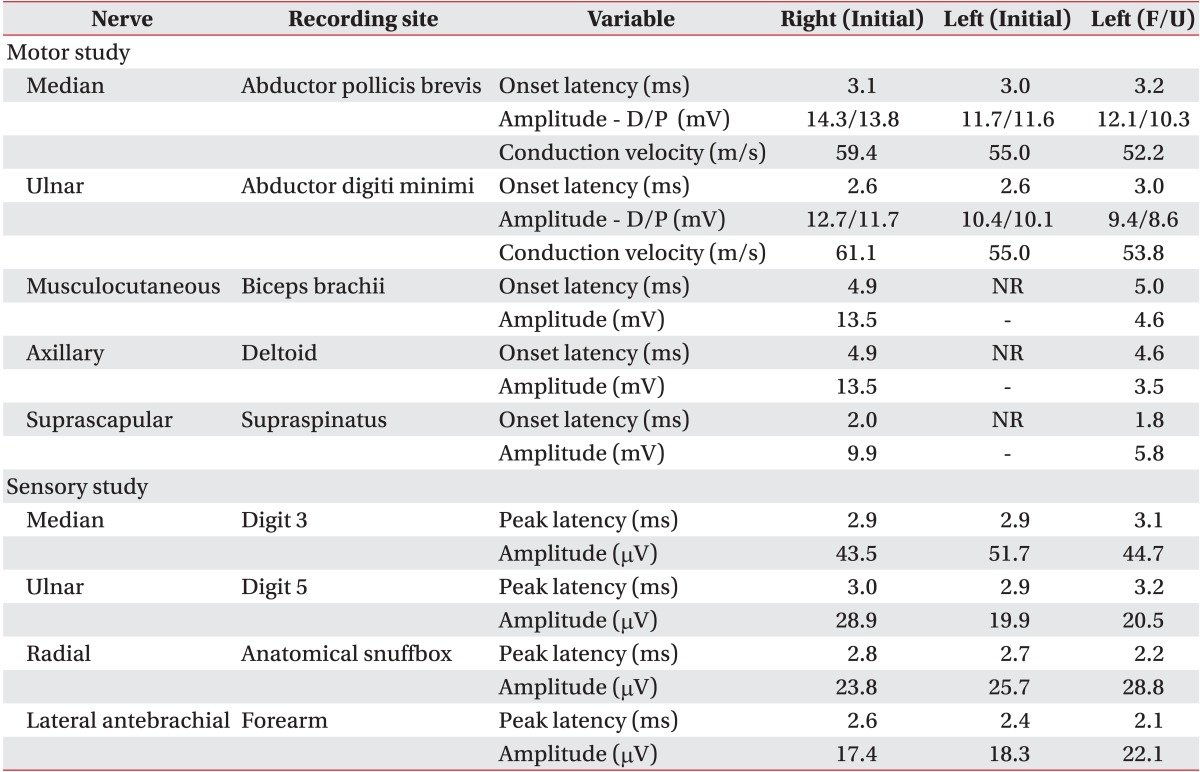

Table 2
Results of left upper extremity electromyography

The patient received physical therapy after discharge. Nine weeks after symptom onset, motor power had improved in the following muscles: left supraspinatus and deltoid was MRC 2/5, left infraspinatus was MRC 3/5, and left biceps and triceps was MRC 5/5. Paresthesia of the left axillary nerve distribution continued. Follow-up electromyography and NCS were performed about 9 weeks after the onset of initial symptoms and showed improved signs of reinnervation. The axillary, musculocutaneous and suprascapular nerves that were absent of CMAPs during the first NCS examination began to show CMAPs, although amplitude was small compared to that of the right side (
Table 1). The MUAPs were polyphasic in the left deltoid and triceps muscles on volition. Interference patterns had improved except that the triceps muscle showed full interference patterns and the supraspinatus, infraspinatus, deltoid, biceps, flexor carpi radialis muscles showed reduced interference patterns. Positive sharp waves had decreased and were observed only in the left infraspinatus muscle (
Table 2).
Go to :

DISCUSSION
Brachial plexus neuritis or neuralgic amyotrophy was first described by the French physician Joffroy in 1879 [
5]. It is characterized by acute onset of severe shoulder pain followed by paralysis of shoulder muscles within days. The exact etiology of brachial plexus neuritis is controversial, though various factors have been postulated including viral infections, trauma, strenuous exercise, recent surgery, immunization, and autoimmune diseases [
6]. Brachial plexus neuritis had been reported with various microorganisms including viruses, bacteria, and molds [
7].
S. agalactiae was originally a causative agent of bovine mastitis but human infections were first reported as postpartum sepsis in 1938. Several cases of sporadic human infection with
S. agalactiae have been reported, although its clinical significance is uncertain. In the 1970s,
S. agalactiae began to show a higher separation rate in newborns and pregnant women. However, it has been recognized as an ever-growing cause of invasive infections in nonpregnant adults more recently [
3].
S. agalactiae infection has become a major problem not only in pregnant women and neonates but also in nonpregnant adults, particularly the elderly or those with chronic disease. Brachial plexus neuritis has been not reported previously in association with
S. agalactiae infection.
The etiology of idiopathic brachial plexus neuritis is thought to be immune mediated, but the precise mechanism is unknown, and little is known regarding the etiology of this disorder. In our case, the supraspinatus and infraspinatus muscles showed positive sharp waves on needle electromyography, indicating damage to the suprascapular nerve at a location proximal to the superior scapular notch. Because the proximal part of the suprascapular nerve and shoulder joint are separated anatomically, the possibility of direct invasion to the nerve is low. Additionally, the abscess was limited to the subdeltoid area on shoulder MRI (
Fig. 1); therefore, nerve compression by the subdeltoid abscess seemed less likely. Approximately 45.0%-53.2% of patients with neuralgic amyotrophy report antecedent events and infections accounted for 43.5% [
2,
8]. After infection, neuralgic amyotrophy occurs within 24 hours in 8.2% and within 1-7 days in 65.3% of patients [
2]. Although the time interval between the infectious event and symptom onset was short in our case, no other causative event was identified except septic arthritis. Thus, the immune reaction may have been triggered by septic arthritis due to
S. agalactiae and, as a result, brachial plexus neuritis occurred.
The most important element for diagnosing brachial plexus neuritis is clinical suspicion based on a detailed history and examination. Up to 5%-15% of patients lack the initial painful stage as in our patient [
8,
9]. This disease is known to involve the shoulder joint most commonly and attacks motor nerves more frequently than sensory fibers, which was consistent with our case [
2,
8]. The overall prognosis in brachial plexus neuritis is good with about 75%-80% of patients achieving functional recovery within 2 years and 90% recover by 3 years [
2,
10]. Our patient's symptoms also improved gradually with 5 months of follow-up. The needle electromyography findings of the patient showed evidence of axonal loss, which is the main finding in cases of brachial plexus neuritis [
8]. Weakness and NCS findings improved during 5 weeks, suggesting that not only axonal loss but also conduction block would have accompanied this disease, although it is difficult to confirm a conduction block in a proximal lesion.
Clinicians should consider the possibility of a preganglionic C5-6 root lesion and mononeuritis multiplex for the differential diagnosis. The initial and follow-up needle electromyography in our case showed no abnormal findings in the cervical paraspinal muscles, the patient had no history of trauma, and showed good recovery. Therefore, preganglionic cervical root lesions seemed to be less likely, as the sensory NCS were normal. The possibility of mononeuritis multiplex involving suprascapular, axillary, and musculocutaneous nerves separately can be ruled out because the triceps and flexor carpi radialis muscles showed axonal degeneration on needle electromyography; thus, it seemed more appropriate to think that the neuritis attacked the upper and middle trunk brachial plexus in a patch fashion.
Neuropathy is a frequent complication of diabetes mellitus, which occurs in approximately 50% of patients with diabetes, but brachial plexopathy has rarely been reported. Up to now, roughly three cases have been reported on brachial plexopathy in patients with diabetes, and in all three cases, patients showed bilateral lesions [
10]. Because our patient showed unilateral brachial plexopathy, the brachial plexus neuritis may not have been caused by diabetes mellitus itself.
The limitation of our case is that a brachial plexus MRI was not performed because of cost. Therefore, the diagnosis was made based on clinical manifestations and electromyography. Lateral antebrachial cutaneous and superficial radial nerves were spared, possibly due to the traits of brachial plexus neuritis, which mainly involve motor nerves and a patch distribution. In addition, although intraneural topography of proximal nerves is not well known yet, funicular patterns of nerves may have contributed to these findings [
8].
In conclusion,
S. agalactiae infection is a major cause of various neurological or medical complications, such as bacteremia with unknown focus, skin and/or soft tissue infections, meningitis, and osteoarticular infection. [
3]. In particular, the incidence of pyogenic arthritis by
S. agalactiae has been increasing [
3]. Infection by
S. agalactiae can lead to various complications, and, as in our case, this organism may also be responsible for brachial plexus neuritis.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download