DISCUSSION
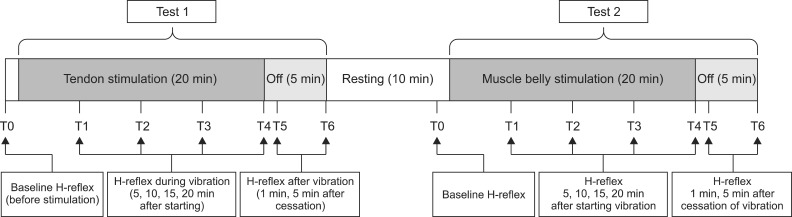
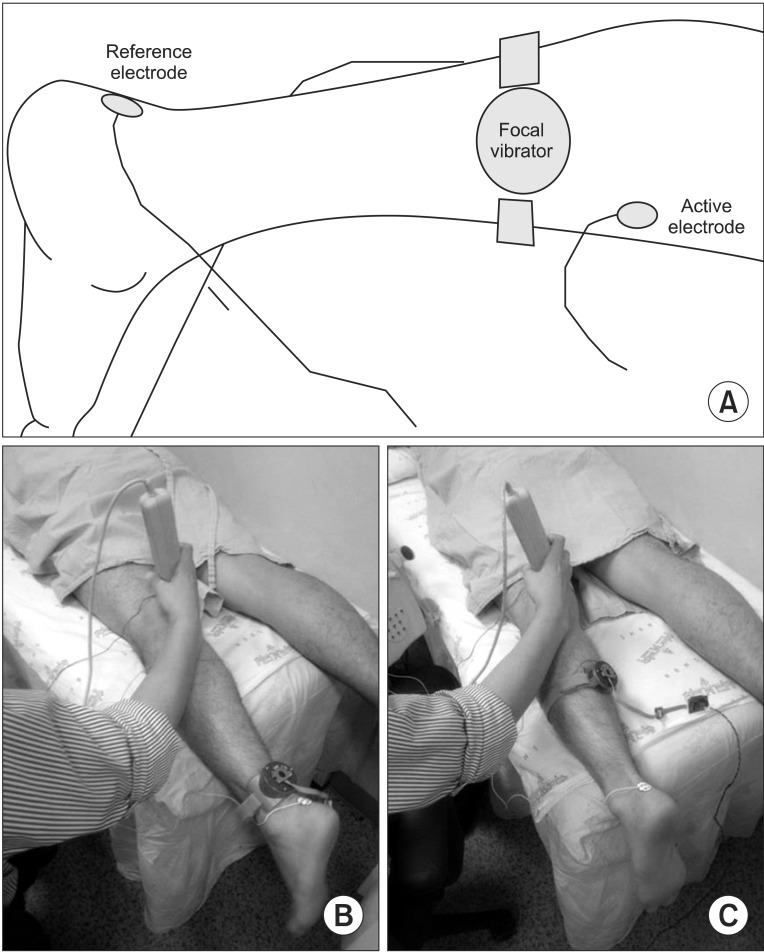
The aim of this study was to verify the difference in electrophysiological effects of a focal vibrator according to the location of stimulation in healthy people. The experiment consisted of 2 subtests that differed solely in stimulation location (muscle belly vs. tendon). All participants completed the 14 consecutive H-reflex evaluations to identify the changes in H-reflex parameters during vibration (20 minutes) and after stimulation (5 minutes). The models using the GEE were established for each of the 4 parameters (MOL, Hmax, HMR, and VII), and statistical analyses were performed.
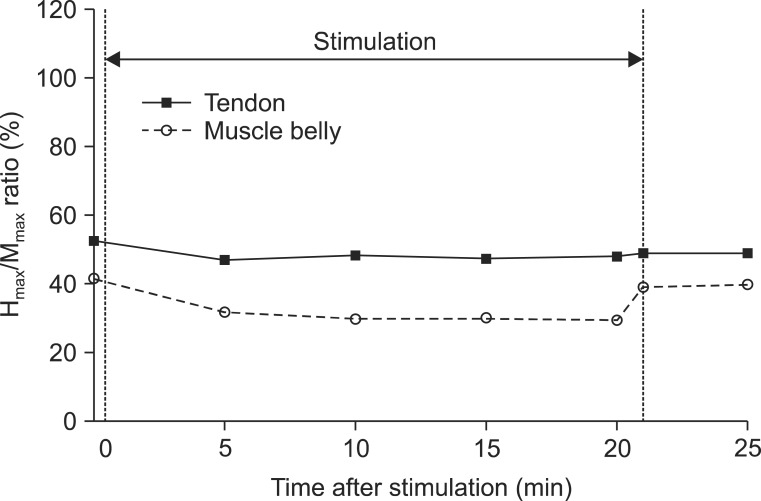
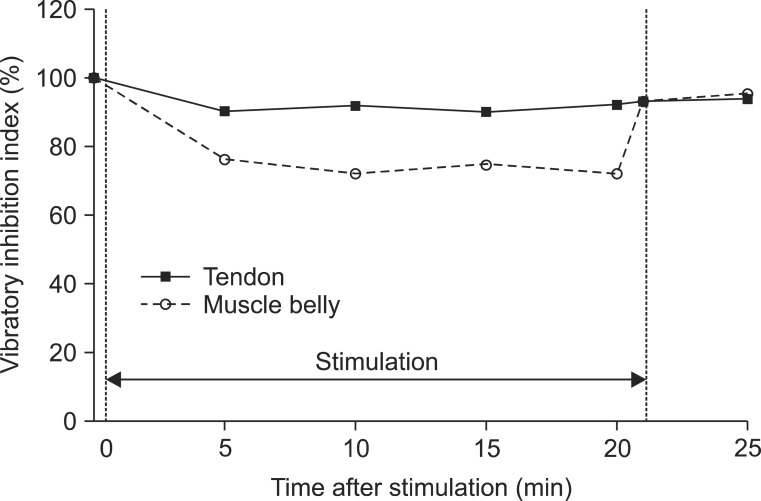
We demonstrated a significantly higher decrease in the Hmax and VII in vibration on the muscle belly than on the tendon, after time correction. We observed and confirmed the changes over time on adjustment of location effect. All parameters except the MOL (Hmax, HMR, and VII) decreased 5 minutes after stimulation, and the decrements were maintained during vibration but recovered immediately after turning off the vibrator.
When vibration is applied to a muscle or tendon, alpha-motoneuron excitability is decreased while the presynaptic inhibition is increased, resulting in reduction in the tone of the stimulated muscle. Previous studies suggested that the decrements in the H
max, HMR, and VII after vibration were correlated with reduced muscle tone and electrophysiological changes [
17,
18]. However, apart from the muscle tone reduction, vibration can also produce the tonic vibration reflex, which usually appears with high frequency and low amplitude stimulation [
15]. The electrophysiological effects of vibration can be varied by frequency or amplitude and posture or the degree of contraction. In this study, 70 Hz with 65 µm vibration on the relaxed gastrocnemius in prone position resulted in decreased motoneuron excitability and presynaptic inhibition, but tonic vibration reflex was not observed.
To verify the difference of vibration location in a muscle, the same protocol was applied to two subtests for the muscle belly and tendon. We selected the gastrocnemius muscle, which is located in a relatively superficial layer, because we thought that the stimulation of a superficial muscle using as low of an energy level as possible was suitable for verifying the effect of focal vibration. Actually, considering that the muscle belly has a higher density of muscle spindles, we can expect the change in the electrophysiological effects to be larger in the muscle belly than in the tendon. The vibration location varied in previous studies that reported a positive effect of vibration on spasticity and related functional impairment. Noma et al. [
12,
19] reported that vibration to the volar side of the fingers, palm, wrist flexor tendon, and muscle belly of the biceps brachii had antispastic effects in the hemiplegic upper limbs of stroke patients. Marconi et al. [
13,
20] suggested that muscle belly stimulation of the flexor carpi radialis and biceps brachii resulted in improving spasticity and motor function with a parallel change in intracortical inhibition. Other studies applying vibration on the tendon area also reported an antispastic effect. The vibration location in the study by Liepert and Binder [
14] was the tendo-muscular passage of the extensor carpi radialis, Conrad et al. [
10] stimulated the tendon area of the wrist flexor, and Caliandro et al. [
21] positioned the vibrator on the distal tendon insertion site. No study has been designed to verify the difference of vibration location in a muscle. Therefore, we focused on investigating the optimal location of vibration in a muscle, which would have a more antispastic effect between the muscle belly and the tendon.
Vibration is the sinusoidal mechanical displacement represented by frequency and amplitude. Vibratory stimulation is known to result in neurophysiological change through mechanoreceptors in the muscles and tendons, such as the muscle spindle and Golgi tendon organ [
22]. Using a miniaturized vibrator for focal stimulation, we measured the parameters of the H-reflex and addressed the differences based on the location of that device. We observed a significantly higher degree of decrease in the H
max and the VII for muscle belly stimulation than tendon stimulation. The HMR also showed a similar difference according to location of the vibration (11.38% lower in muscle belly stimulation) with moderate significance (p=0.10). This difference is thought to be due to the distribution of the mechanoreceptors in the muscle. The muscle spindles mainly distribute within the deeper muscle regions according to the branching pattern of the neurovascular bundle [
23]. Conversely, the Golgi tendon organs are known to be located relatively near tendons [
24]. Among various mechanoreceptor networks, the Ia afferent fiber population response via muscle spindles is likely to be the largest [
22]. In this study, muscle belly stimulation, where the density of muscle spindles is relatively high, elicited larger changes in the H-reflex parameters than tendon vibration. Though other muscles are expected to have a similar response, optimization of the vibration location may be necessary. The distribution of mechanoreceptors is dependent on the type of muscle [
25].
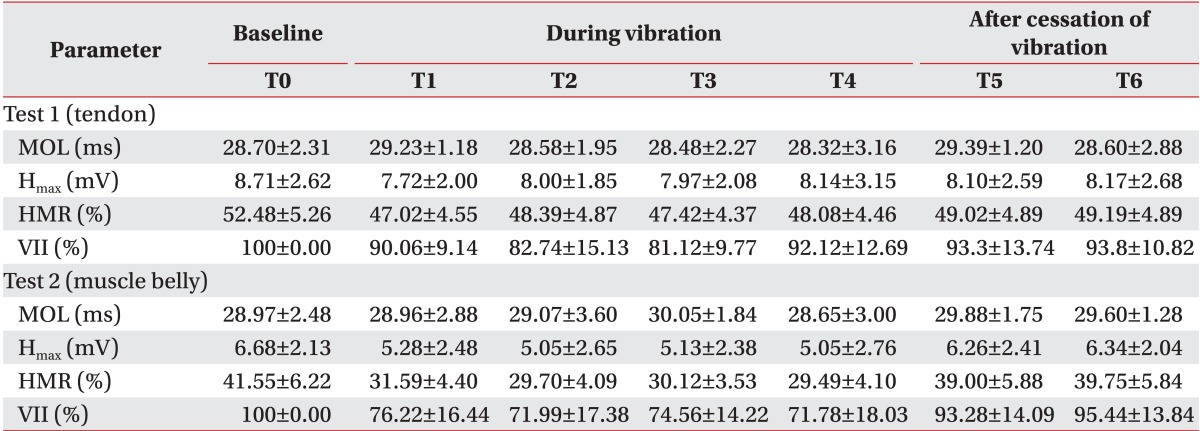
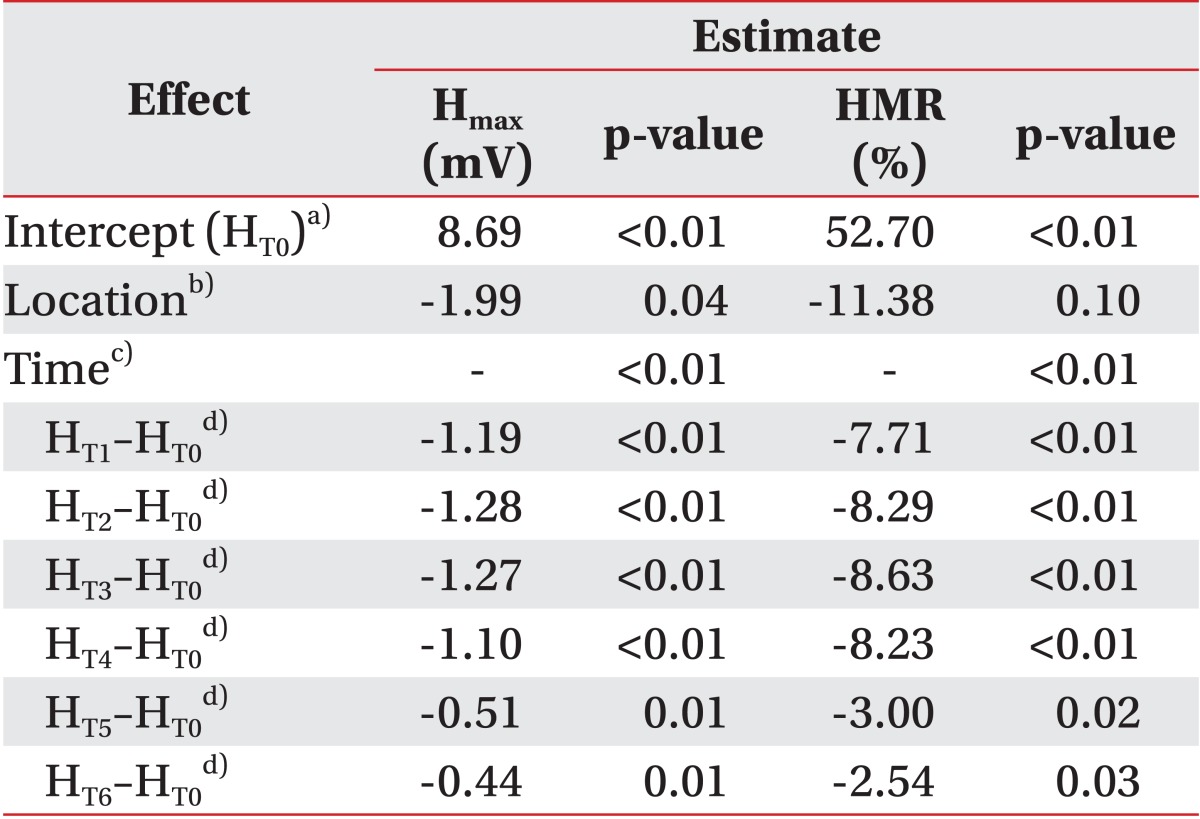
We also performed a comparison of the baseline between test 1 and test 2 using the Wilcoxon signed-rank test. The H
max and HMR before test 2 were significantly lower than before test 1, 10.93% (79.17% of test 1, p=0.01) and 2.03 mV (76.69% of test 2, p<0.01), respectively. In contrast, the MOL did not show any significant difference (p=0.08) (
Table 1). The mean H
max before test 2 (6.68 mV) was smaller than that after test 1 (T6, 8.17 mV, p=0.04). Therefore, the residual effect of vibration from test 1 affecting test 2 should be considered. However, the long-term effect had minimal impact on the main findings of this study and their interpretation as follows: firstly, previous studies reported that the inhibitory effect on the H-reflex from vibration disappeared quickly. The experiments of van Boxtel [
17], who conducted consecutive measurements of the H-reflex during vibration, showed intermittent depression during 2 minutes of stimulation and immediate recovery after stopping vibration near the baseline. In this study, we designed a 15-minute washout period, which included 5 minutes after stopping vibration in test 1 and a 10-minute interval between the subtests. We judged that it was sufficient to dilute the effect of previous vibration. Secondly, the results of test 1 after vibration already showed almost full recovery. For example, the H
max of test 1 at T5 and T6 was restored to 93.3% and 93.8% of T0, respectively. The baseline of test 2 was below 80% of that in test 1, so that residual effect could not explain this difference. Lastly, the possible effects on the results of this study from the baseline differences of parameters between the two subtests were controlled using statistical methods. We set the statistical models of the MOL, H
max, and HMR using the baseline parameters as a covariate, so that larger decrease of the H
max and HMR was found in muscle belly vibration (
Table 2). We also confirmed the location effect on the VII, which was originally adjusted for the baseline in each subtest (χ
2=4.54, p=0.03). Considering these explanations, the baseline difference between the two subtests was due to another cause. The most plausible reason would be the location change of the vibrator. When the location was shifted from the Achilles tendon to the calf, the silhouette of soft tissue and muscle around the vibrator differed and the recording electrode might have changed inevitably. To eliminate this conformational change between the subtests, we plan to conduct the experiments in random order after the installation of two vibrators on the tendon and muscle belly in advance.
The immediate electrophysiological response to vibration was found in both the tendon and muscle belly stimulation test. We can confirm the depression of the H-reflex parameters not only at 5 minutes after starting vibration but also at 1 minute after holding the stimulation. These results are compatible with previous studies [
17,
26]. Conversely, the degree of change in this study (20%-30%) was relatively smaller than in the previous studies (40%-50%) [
27]. Several factors, such as the muscle type and parameters of vibration, may be related to the difference. The muscle stimulated in most other studies was the soleus [
27], but the gastrocnemius was stimulated in this study. As previously mentioned, the distribution of mechanoreceptors differs among the types of muscles [
25], accordingly the electrophysiological response to specific stimulation is expected to be different. The change in the H-reflex from vibratory stimulation is known to have correlated with the parameters of vibration [
28]. The frequency and amplitude used in this study would have produced a relatively lower energy compared to other studies. We selected the appropriate parameters, which were safe and energy efficient, and elicited the sufficient electrophysiological response simultaneously. Although the decrease was smaller than in previous studies, we verified that 70 Hz and 65 µm vibration led to a significant H-reflex change.
About 30 years ago, Lance [
29] defined spasticity as a motor disorder characterized by a velocity-dependent increase in muscle tone, resulting from hyperexcitability of the stretch reflex, which is known as the representative clinical manifestation of the upper motor neuron disease (e.g., stroke and spinal cord injury). The definition is based on the classical model of spasticity related to muscle spindle, spinal reflex circuit, and the upper motor neuron. Recently, Lance's definition was determined to be limited, because hyperexcitability and motor dysfunction is not matched on occasion. However, it is still widely operational in clinical and research fields that focus on the pathophysiology of spasticity through neurophysiological parameters. The pathophysiology of spasticity derived from recent research can be summarized as follows: 1) a decreased homosynaptic depression at the synapse between the Ia afferent and the motoneuron; 2) changes in the motoneuron properties; and 3) changes in the muscle properties [
30].
Reliable and valid assessment of spasticity is essential for experimental and clinical purposes. Many researchers have investigated electrophysiological methods for objective quantitation of spasticity. For example, the H-reflex, F-wave, and electromyographic recordings during movement evoke potential. Among these tests, the H-reflex is the most representative indicator of spasticity, known as the electrical analog of the tendon stretching reflex. It reflects the monosynaptic reflex arc, which consists of sensory afferent fiber (Ia) and alpha-motoneurons [
17]. The parameters related to the H-reflex, such as the H
max, MOL, HMR, VII, are frequently used in many studies [
27]. It is well established that the change in the H-reflex parameters represents the electrophysiological aspects of spasticity, such as the alpha-motoneuron excitability, presynaptic inhibition, reciprocal inhibition, recurrent inhibition, and polysynaptic change [
27,
31]. While the degree of change differs on each report, the results of the H-reflex from a patient with spasticity have demonstrated an overall increase in H
max, HMR, and VII and a decrease in MOL [
27].
The H
max is an expression of alpha-motoneuron excitability to excitatory inputs from Ia-afferents [
31]. We observed a significant decrease in the H
max during vibration, and this direction of electrophysiological change may alleviate spasticity. The HMR is used in many studies in order to compensate the variability of the H
max based on individual characteristics [
32]. In this study, the HMR was also reduced significantly during vibratory stimulation. Furthermore, we investigated VII, which is the ratio of the H
max during vibration to the H
max before vibration. An inverse correlation between the VII and presynaptic inhibition has been reported [
18]. VII is known to be increased in upper motor neuron disease, which results in reduction of presynaptic inhibition, and it is correlated with the degree of spasticity [
33]. Similar to other H-reflex amplitude related parameters, the VII also showed a decrease during vibration, and this change can also lead to reduced spasticity. However, we could not find a significant difference in the MOL of the H-reflex related to vibration or not and the location of stimulation. This may be because the MOL has a larger inter-individual variability and a smaller inter-rater reliability than the H-reflex amplitude-related parameters [
34]. The correlation between the MOL and spasticity showed inconsistent results in previous studies. Some researchers reported that a decreased MOL was found in spastic subjects, indicating higher excitability of the motoneuron pool [
35]; however, in other studies, values that approach normal levels were also found [
36]. In future studies, it would be favorable to use the parameters related to the H-reflex amplitude.
Actually, the neurophysiological effects of vibration were not limited to the level of the spinal cord and below. Vibratory stimulation is a strong proprioceptive stimulus and can reach both the primary somatosensory and motor cortices [
37,
38,
39] and change the intracortical inhibitory circuit [
40]. Several studies proposed that the vibration in patients with spasticity can lead to the redistribution of intracortical synapses related to supraspinal motoneuron excitability control, and the effects would be correlated with long-term functional improvement [
17,
18,
19,
20]. Vibration can also mechanically mobilize the muscle and connective tissue to alleviate stiffness and contracture. Thus, vibratory stimulation can play an important role in improving the spasticity at different levels of the neuromuscular system.
Recently, many studies have reported using vibratory stimulation to improve spasticity in stroke patients. Depending on the extent of vibration, the type of vibratory method in these studies has been categorized as whole body vibration, segmental vibration, and focal vibration. Whole body vibration transfers the vibratory stimulation to the whole body by using a vibrating platform with the patient in standing or sitting position [
8,
9]. Segmental vibration can be defined as the way of stimulation in several muscles or tendons with one or several vibrators, though the term has been frequently confused with focal vibration [
11]. In contrast to segmental vibration, focal vibration implies a stimulation method with a narrow range of spread, which could vibrate just one muscle or tendon [
10,
14,
21]. The attempt to improve spasticity using whole body vibration has been unsuccessful to date [
8,
9]. Contrarily, the studies that applied segmental or focal vibration have reported positive results on motor function, improvement of spasticity, and other neurophysiological changes [
10,
11,
12,
13,
14,
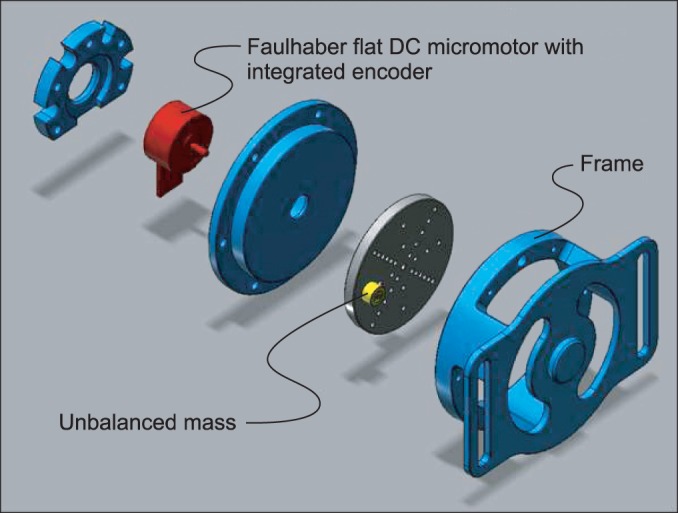
21]. Compared to whole body vibration, segmental or focal vibration can be targeted to the muscle with spasticity, resulting in less systematic side effects, such as nausea and dizziness. Through miniaturization of the vibrator, its simultaneous application with other therapies or during everyday life activities is possible. However, the concept of focal or segmental stimulation improving spasticity still lacks clinical evidence, because the number of participants in each study was small and the factors affecting outcomes (i.e., location, parameters of the vibration, and the clinical setting) were not controlled or appropriately compared. The vibrators in previous studies also had some shortcomings for investigating optimal conditions of focal or segmental vibration in various clinical settings. The part that transmitted the vibration was small, but the entire apparatus was too large and heavy to move to other places, adjust to other positions, and apply during other physical therapy treatments. Alternatively, the newly developed focal vibrator used in the current study is light and small. The alteration of vibration parameters and programmable stimulation at multi-sites is possible with a remote control. We expect that a portable vibrator like this would be helpful for investigating the optimal parameters of vibration for improving spasticity.
This study had some limitations that mostly stemmed from its small sample size. We focused on the electrophysiological difference based solely on the vibration location in a muscle. However, future studies on the optimal frequency and amplitude of vibration for improving spasticity have been planned. To confirm the extent of vibrational spread, the outcome measurements in the neighboring and antagonist muscles to the vibrated muscle, are essential. To completely prove the effect of focal vibration on the motor control system, the changes in the central nervous system above the spinal cord level, in the muscle itself, and in the soft tissue should be evaluated. To verify the clinical usefulness of this focal vibrator on spasticity, we need to evaluate the neurophysiological effects in patients with spasticity and to analyze the correlation between electrophysiological changes and functional improvements.
Despite these limitations, we confirmed significant implications for the clinical application of a miniaturized focal vibrator. Firstly, the focal vibratory stimulation had no severe adverse effects and was relatively safe compared to other therapeutic interventions, such as medication and botulinum toxin injection. Secondly, the defined vibratory stimulation on the belly of a specific muscle was possible, and the selective improvement of spasticity was expected. Although the clinical manifestation of spasticity in stroke patients usually has a synergistic pattern, the target of most antispasticity therapy is not the entire muscle with spasticity but the specific muscle that is important for improving the function or symptom. Additionally, spasticity in only one muscle, such as the striatal toe, which is selective tone increase in the extensor hallucis longus muscle, was also found. We expect functional improvements in stroke patients through individualized muscle tone management using a focal vibrator that can selectively stimulate specific muscle. Finally, the newly developed vibrator is portable, so that ADLs and rehabilitation therapy can be performed during application. Range of motion exercise or gait training with simultaneous vibration is thought to be more effective. Furthermore, we expect a portable vibrator for embedded functional orthosis to be developed, which will modulate the muscle tone in real-time.
In conclusion, the neurophysiological surrogate markers of spasticity (Hmax, HMR, and VII) immediately responded to vibrator on and off, reduced, and recovered, respectively. During vibration, the degree of the effect was unchanged. The effect size of the vibration was larger in the muscle belly than in the tendon. Further studies in stroke patients using a focal vibrator are essential to address the selective vibration effect on the improvement of spasticity, gait function, and ADLs.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download