Abstract
It can be difficult for clinicians to distinguish a paradoxical response to antituberculous therapy, worsening of an existing lesion despite adequate treatment, treatment failure, and drug resistance. We report a case of a 69-year-old woman who experienced bilateral lower extremity paralysis secondary to a paradoxical response. She had been suffering for 1 month from low back pain, due to tuberculous spondylitis. Her low back pain improved after antituberculous therapy. The low back pain, however, reappeared 2 months after treatment, accompanied by newly developed lower extremity weakness. Imaging studies showed an increased extent of her previous lesions. Consequently, the patient underwent a vertebral corpectomy with interbody fusion of the thoracolumbar spine. Histopathological examination showed chronic inflamed granulation tissue with no microorganisms. Although the antituberculous medication was not changed, the patient's symptoms and signs, including the paralysis, resolved after surgery.
Tuberculous spondylitis is a common extrapulmonary manifestation of tuberculosis. Patients with tuberculous spondylitis usually respond well to medical treatment [1]. A paradoxical response is defined as worsening of existing symptoms or the appearance of a new lesion in a patient who initially responded well to antituberculous therapy [2]. A paradoxical response involving the spine or paraspinal structures may develop when the initial lesion is in the spine [3], and also when the primary lesion is elsewhere [4,5]. A paradoxical response should be differentiated from treatment failure, misdiagnosis, and poor compliance. Sometimes, it is especially difficult to differentiate a paradoxical response involving the initial presentation site from treatment failure by radiologic findings. In this report, we present a case of a paradoxical response that developed at the site of the initial tuberculous spondylitis, causing spinal cord compression, with a review of the literature.
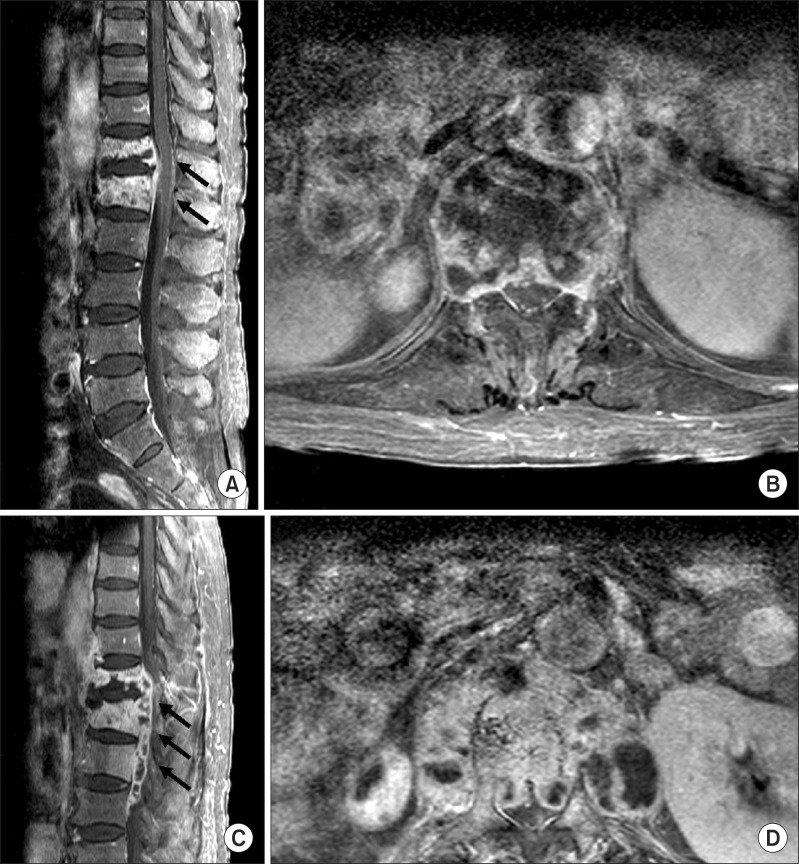
A 69-year-old woman with a 1-month history of back pain was admitted to a tertiary hospital. Although she had received supportive care at a community hospital, her low back pain was aggravated to 10 points on a numerical rating scale (NRS; scale from 0 to 10), and general weakness, weight loss (10 kg), and fever were evident. In a neurological examination, motor and sensory deficits were indefinite in the bilateral lower extremities (Table 1). Bilateral hip flexors could not be checked due to pain. There was no voiding or bowel symptoms. Furthermore, deep tendon reflexes were normoactive in the bilateral lower extremities, with no upper motor neuron signs. Laboratory findings in peripheral blood showed an elevated erythrocyte sedimentation rate (ESR) of 85 mm/hr and C-reactive protein (CRP) of 1.86 mg/dL. However, white blood cell counts (5,510/µL) and the percentage of lymphocytes (21.1%) were normal. She denied a previous history of tuberculosis infection. The screening test for human immunodeficiency virus (HIV) was negative. Spinal magnetic resonance imaging (MRI) showed a low signal intensity at T12 and the L1 vertebral body in a T1-weighted image with gadolinium enhancement, suggesting paravertebral abscess formation (Fig. 1A, B). Diffuse, tiny centrilobular nodules and branching opacity with ground glass opacity in both lungs, suggestive of miliary tuberculosis, were detected on chest CT. Mycobacterium tuberculosis complex, susceptible to all first-line antituberculous drugs, was isolated from the bone biopsy of the L1 vertebral body, confirming the diagnosis of tuberculous spondylitis with miliary tuberculosis. Antituberculous therapy including isoniazid 300 mg, rifampicin 600 mg, and ethambutol 800 mg once per day (HRE) was started. After 1 week of HRE therapy, the medication was changed to ethambutol 800 mg, amikacin 500 mg, and levofloxacin 500 mg daily for 1 month, and then changed to isoniazid 300 mg, ethambutol 800 mg, and levofloxacin 500 mg per day due to hepatotoxicity. She was transferred to department of rehabilitation medicine for managing her low back pain, spinal brace, and gait training. The low back pain and general weakness improved after 1 month of antituberculous therapy. Her low back pain was then scored as 3 points with the NRS. Her chest X-ray showed improvement. She was transferred to a convalescent hospital for long-term management.
Then 2 months after antituberculous therapy began, low back pain was aggravated to 7 points on the NRS despite pain medication. Furthermore, weakness in the bilateral lower extremities developed with no trauma history. She visited the outpatient rehabilitation clinic at 3 months after antituberculous chemotherapy began to manage her aggravated symptoms. She denied discontinuation of the antituberculous medications. On a neurological examination, new weakness in the lower extremities had developed (Table 1). Pin-prick sense was decreased at the T12 level and below, and light-touch sense was decreased at the L1 level and below. Anal sensation and anal sphincter power were intact. There was no voiding or defecation symptom. Imaging studies revealed an increased extent of the lesions at the vertebral bodies and paravertebral structures, including the epidural space, compressing the spinal cord (Fig. 1C, D). Blood tests showed an elevated ESR of 51 mm/hr and CRP of 3.19 mg/dL. There was no surge in lymphocytes (white blood cell counts 4,020/µL, percentage of lymphocytes 18.2%). Considering the duration of the antituberculous therapy without drug discontinuation, a paradoxical response was suspected.
The patient underwent a T12-L1 vertebral corpectomy and T11-L2 interbody fusion, considering her progressive neurologic symptoms and instability of the spine. Puncture of the abscess cavity in left psoas muscle released a clear fluid, which was not suggestive of a cold abscess. In the ventral epidural space, there were only inflamed tissues, with no caseous material, again suggesting a paradoxical response. On microbiological and histopathological examinations of the surgical specimens, there was chronic granulomatous inflammation with necrosis, with no microorganisms. Her antituberculous medication was maintained without change. After the surgery, the patient's symptoms of lower extremity weakness and low back pain improved (Table 1). She was able to walk without assistance or a device at 6 months after the operation.
In this case, the patient suffered from low back pain due to tuberculous spondylitis with no neurological deficit. The antituberculous medications were successful in treating the spondylitis. Worsening of the back pain and neurologic deficits, however, developed 2 months after the treatment. It was impossible to differentiate a paradoxical response from treatment failure from the MRI images, which simply showed an increased extent of the lesion. A paradoxical response was suspected according to the clinical features, and was eventually confirmed by surgical exploration and histopathological examination.
Cheng et al. [3] reported the prevalence of paradoxical responses to be 15.4% (16/104 patients) and the presentation of a paradoxical response in tuberculous spondylitis as low back pain in five HIV-negative patients with no motor symptoms. Cheng et al. [2] reviewed 122 episodes of paradoxical responses after antituberculous therapy in HIV-negative patients and described 17 episodes of motor dysfunction caused by brain lesions, not spinal lesions. Since that literature review, several reports [4,5] have been published describing myelopathy induced by paradoxical responses. In those reports, paradoxical responses developed as intradural extramedullary tuberculomas at a site remote from the initial infection site. Takahashi et al. [4] reported a patient with tuberculosis meningitis who developed paraplegia due to a paradoxical response involving an intradural extramedullary tuberculoma of the upper thoracic spinal cord after 8 weeks of antituberculous treatment. Muthukumar et al. [5] reported a similar case of thoracic myelopathy, developed as a paradoxical response with an intradural extramedullary tuberculoma of the lower thoracic spinal cord 3 months after antituberculous treatment for meningitis. However, our report described paraplegia resulting from a paradoxical response that developed at the site of the initial infection (i.e., tuberculous spondylitis), not involving an intradural extramedullary tuberculoma. This made the differential diagnosis by MRI between a paradoxical response and treatment failure more difficult.
The pathogenesis of paradoxical responses is thought to be related to the immune-mediated response. Transforming growth factor-β induced by purified protein derivative from M. tuberculosis suppressed host T-cell and macrophage activity [6]. After antituberculous therapy, immune restoration may occur rapidly, restoring T-cell function and the secretion of inflammatory cytokines, such as interferon-γ and tumor necrosis factor-α [7]. However, much of the pathophysiological process of paradoxical responses remains unknown. The median time of onset after antituberculous therapy is approximately 56 days (range, 20-109 days) [3]. Paradoxical responses are more common in patients with tuberculosis and HIV coinfection [8]. Risk factors for paradoxical responses in HIV-negative patients are extrapulmonary involvement in the initial tuberculosis, lower baseline lymphocyte counts, and a surge in lymphocyte counts during the paradoxical response [2,3]. There is no established diagnostic method to differentiate a paradoxical response from treatment failure. Blumberg et al. [9] suggested that the paradoxical response should be diagnosed by excluding other etiologies, especially treatment failure. Additionally, treatment failure should be considered when continued or recurrent cultures are positive after 4 months of antituberculous therapy. Treatment failure can be caused by several factors, such as drug discontinuation, malabsorption of a drug, or laboratory errors [9]. In this case, newly developed paraplegia occurred before 4 months and the patient took her antituberculous medications appropriately. Thus, we suspected a paradoxical response rather than treatment failure.
There is no standard management for a paradoxical response. The systemic use of steroids without changing the antituberculous therapy may be effective and safe [2,10]. However, there has been no randomized controlled trial regarding systemic steroid use in paradoxical responses. Surgical decompression may be indicated for tuberculous spondylitis patients with progressive neurologic symptoms. In this case, the patient underwent surgical decompression and spinal fusion without changing the antituberculous medication. The outcome of surgery was favorable.
In summary, this case indicates that physicians should consider a paradoxical response when deteriorating symptoms develop in tuberculosis patients at approximately 2 months after adequate antituberculous treatment. In a paradoxical response, changing the antituberculous medication is not necessary. Surgical decompression should be considered for progressive neurologic deficits. We report here paralysis secondary to a paradoxical response, which developed at the site of tuberculous spondylitis, and a review of the literature.
References
1. Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med. 2011; 34:440–454. PMID: 22118251.

2. Cheng VC, Ho PL, Lee RA, Chan KS, Chan KK, Woo PC, et al. Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2002; 21:803–809. PMID: 12461590.

3. Cheng VC, Yam WC, Woo PC, Lau SK, Hung IF, Wong SP, et al. Risk factors for development of paradoxical response during antituberculosis therapy in HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2003; 22:597–602. PMID: 14508660.

4. Takahashi H, Ito S, Kojima S, Tanno T, Hattori T. Intradural extramedullary tuberculoma of the thoracic spine: paradoxical response to antituberculous therapy. Intern Med. 2008; 47:797–798. PMID: 18421202.

5. Muthukumar N, Sureshkumar V, Ramesh VG. En plaque intradural extramedullary spinal tuberculoma and concurrent intracranial tuberculomas: paradoxical response to antituberculous therapy: case report. J Neurosurg Spine. 2007; 6:169–173. PMID: 17330587.
6. Toossi Z, Young TG, Averill LE, Hamilton BD, Shiratsuchi H, Ellner JJ. Induction of transforming growth factor beta 1 by purified protein derivative of Mycobacterium tuberculosis. Infect Immun. 1995; 63:224–228. PMID: 7806361.

7. Marshall BG, Chambers MA. Central nervous system tuberculosis: the paradox of the host immune response. J Infect. 1998; 36:3–4. PMID: 9515661.
8. Lorent N, Sebatunzi O, Mukeshimana G, Van den Ende J, Clerinx J. Incidence and risk factors of serious adverse events during antituberculous treatment in Rwanda: a prospective cohort study. PLoS One. 2011; 6:e19566. PMID: 21611117.

9. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003; 167:603–662. PMID: 12588714.
10. Bukharie H. Paradoxical response to anti-tuberculous drugs: resolution with corticosteroid therapy. Scand J Infect Dis. 2000; 32:96–97. PMID: 10716088.

Fig. 1
Gadolinium-enhanced fat-suppressed T1-weighted magnetic resonance images of the spine. Before antituberculosis therapy, images revealed low signal changes in the T12 and L1 vertebra body with intense enhancement in the sagittal (A) and axial planes (B). At 95 days after appropriate anti-tuberculosis therapy, images showed increased T12-L3 inflamed tissue, resulting in more central canal compromise and cord compression in the sagittal (C) and axial planes (D). Arrows indicate key findings.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download