Abstract
Objective
To analyze association between urodynamic study (UDS) parameters and renal function in spinal cord injured (SCI) patients with neurogenic detrusor overactivity.
Methods
Patients with a suprasacral SCI, who underwent UDS and radioisotope renogram at least twice between January 1, 2006 and January 31, 2013, were included. UDS (cystometric capacity, reflex volume, compliance, and maximal detrusor pressure) and radioisotope renogram (total effective renal plasma flow [ERPF] of both kidneys) data were collected. The following were conducted to reanalyze any association between reflex volume and ERPF: initial and follow-up results of consecutive evaluations were compared; a mixed-model regression analysis to account for clustered data was conducted to evaluate the association between UDS parameters and ERPF; and finally, a mixed-model analysis type 3 test with data pairs, of which the first evaluation showed involuntary detrusor contraction.
Results
A total of 150 patients underwent 390 evaluations which were arranged into 240 pairs of consecutive evaluations, of which 171 had first evaluations with observed involuntary detrusor contraction. The following results were obtained: cystometric capacity was significantly larger and maximal detrusor pressure was significantly lower on follow-up; on univariate analysis, reflex volume and maximal detrusor pressure were significant, and multivariate analysis using these two parameters showed that maximal detrusor pressure is significantly associated with total ERPF; and no significant differences were observed.
Go to : 
Neurogenic bladder is a common complication in spinal cord injured (SCI) patients [1]. Neurogenic detrusor overactivity can cause renal function deterioration and renal failure [2] which used to be one of leading causes of mortality in SCI patients [3,4]. Therefore, the most important goal in the treatment of neurogenic bladder in SCI is the prevention of upper urinary tract complications and the maintenance of renal function [5].
Urodynamic study (UDS) has been recommended as the gold standard for the evaluation of lower urinary tract function [6], and regular follow-up of UDS was stated to be mandatory for the maintenance of renal function [7].
Previous studies have revealed an association between some of the UDS parameters (i.e., intravesical leak point pressure, detrusor pressure, compliance, maximum urethral pressure gradient [MUPG], duration of uninhibited bladder contraction) [8,9,10,11,12,13,14]. However, most of these studies detected upper urinary tract complications by only assessing its morphological changes, thus any direct assessments of renal functions are lacking. In one study, upper urinary tract stasis was used to find any association between UDS parameters with upper urinary tract complications. Yet, commonly abnormality in renal function precedes morphological changes of the upper urinary tract, so upper urinary tract complication should be detected at an earlier stage [15,16,17,18]. This is why we assumed analyzing the association of UDS parameters with renal function evaluations instead of morphological changes of upper urinary tract would detect any association more sensitively.
Go to : 
The patients included in this study were in- and out-patients with SCI at a single university hospital, who underwent UDS and radioisotope renogram using technetium-99m mercaptoacetyltriglycine (Tc-99m MAG3), after signing a written consent form at least twice between January 1, 2006 and January 31, 2013. Each patient underwent neurological examination according to the American Spinal Injury Association impairment scale. Only the patients with neurogenic detrusor overactivity caused by a suprasacral lesion (without an accompanied sacral lesion) were included. The exclusion criteria were history of any urologic disorders or decline of renal function prior to the SCI, combined traumatic brain injury, language or cognitive impairment which inhibits following on-step verbal commands, and failure of UDS.
This study was approved by the medical ethical committee at our hospital.
Two urodynamic systems were used in this study. From January 1, 2006 up to February 25, 2010, UDS was performed with Duet Encompass (Mediwatch, Rugby, UK). On February 26, 2010, the urodynamic system was switched to Duet Logic G2 (Mediwatch), which was used to perform UDS during the rest of the study period.
With either urodynamic system, the patient was in supine position and normal saline at room temperature was infused into the bladder through a double lumen catheter at a rate of 30 mL/min. The abdominal pressure was recorded via a rectal balloon catheter. The following four parameters were collected for this study: cystometric capacity, reflex volume, compliance, and maximal detrusor pressure. Cystometric capacity was defined as the bladder volume at the end of the filling cystometrogram. Bladder filling was ended if the patient showed leakage, if the patient reported urgency, which made further infusion difficult, or if 450 mL of normal saline had been infused. Reflex volume was defined as the bladder volume at which involuntary detrusor contraction was first observed. Compliance was defined as the relationship between change in bladder volume and change in detrusor pressure, and was calculated by dividing the volume change by the change in detrusor pressure during that change in bladder volume. Maximal detrusor pressure was defined as the maximum value of detrusor pressure during the filling cystometrogram.
Radioisotope renogram using Tc-99m MAG3 was carried out with Vertex EPIC (ADAC, Milpitas, CA, USA), and effective renal plasma flow (ERPF) for each kidney and the sum of both were collected for this study.
Data collected was encoded and statistical analysis was performed with SAS statistical software package ver. 9.3 (SAS Institute, Cary, NC, USA).
First of all, the frequencies, means, and standard deviations were determined for the baseline characteristics of the study population. For the second and third analyses, the data were arranged into pairs of consecutive evaluations. As patients underwent evaluations different number of times during the study period (two to six times), and as they suffered SCI at different points of time before the study, for statistical analysis, the change between two consecutive evaluations was considered independent from SCI onset duration, and from change between previous or later evaluations. The data were uniformly arranged into pairs of two consecutive evaluations, regardless of the interval between each two evaluations. Three or more consecutive evaluations of the same patient were divided into several pairs of consecutive evaluations, and all pairs were included in the data set. Thus, a total of 150 patients underwent a total of 390 evaluations, which yielded 240 data pairs.
In UDS evaluations, in which involuntary detrusor contraction was not observed, reflex volume was not available. Instead of performing the second and third statistical analyses with missing data, we inserted the corresponding value of cystometric capacity as reflex volume in order to increase the accuracy of the statistical analyses.
So, each data pair consisted of initial and follow-up results of four UDS parameters and a total ERPF. For the second analysis, a paired sample t-test of the initial and follow-up results was performed at a significance level of p<0.05.
Then, for the third analysis, the study outcomes were analyzed by mixed-model regression techniques to account for clustered data. To assess the relationship of the four UDS parameters to total ERPF, a univariate analysis was performed; and then, using the significant parameters, a multivariate analysis was performed.
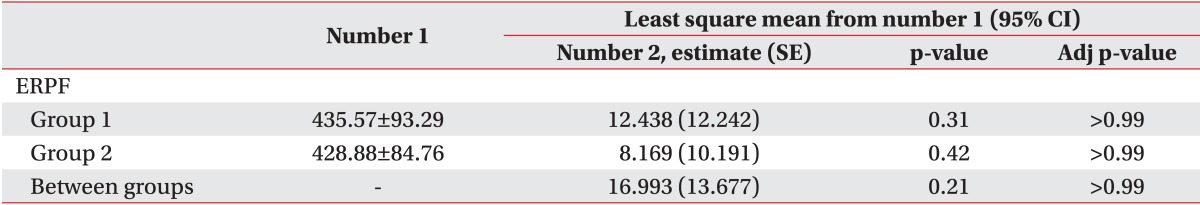
Finally, we considered the possibility that replacing the missing values of reflex volume with corresponding values of cystometric capacity may have rendered reflex volume as falsely not significant. Thus, for the fourth analysis, only the data pairs were selected, in which the first evaluation showed involuntary detrusor contraction. The data pairs were then categorized into two groups: one group with follow-up reflex volume lower than the initial value and the other group either with greater follow-up reflex volume or without observed involuntary detrusor contraction, at follow-up. ERPF, a repeatedly measured variable, was analyzed using a linear mixed-model for numerical measures with fixed effect and random effect. When the interaction group, time, group by time of the variable showed statistical significance, post-hoc analysis was done with Boferroni correction for the adjustment for multiple comparisons.
Go to : 
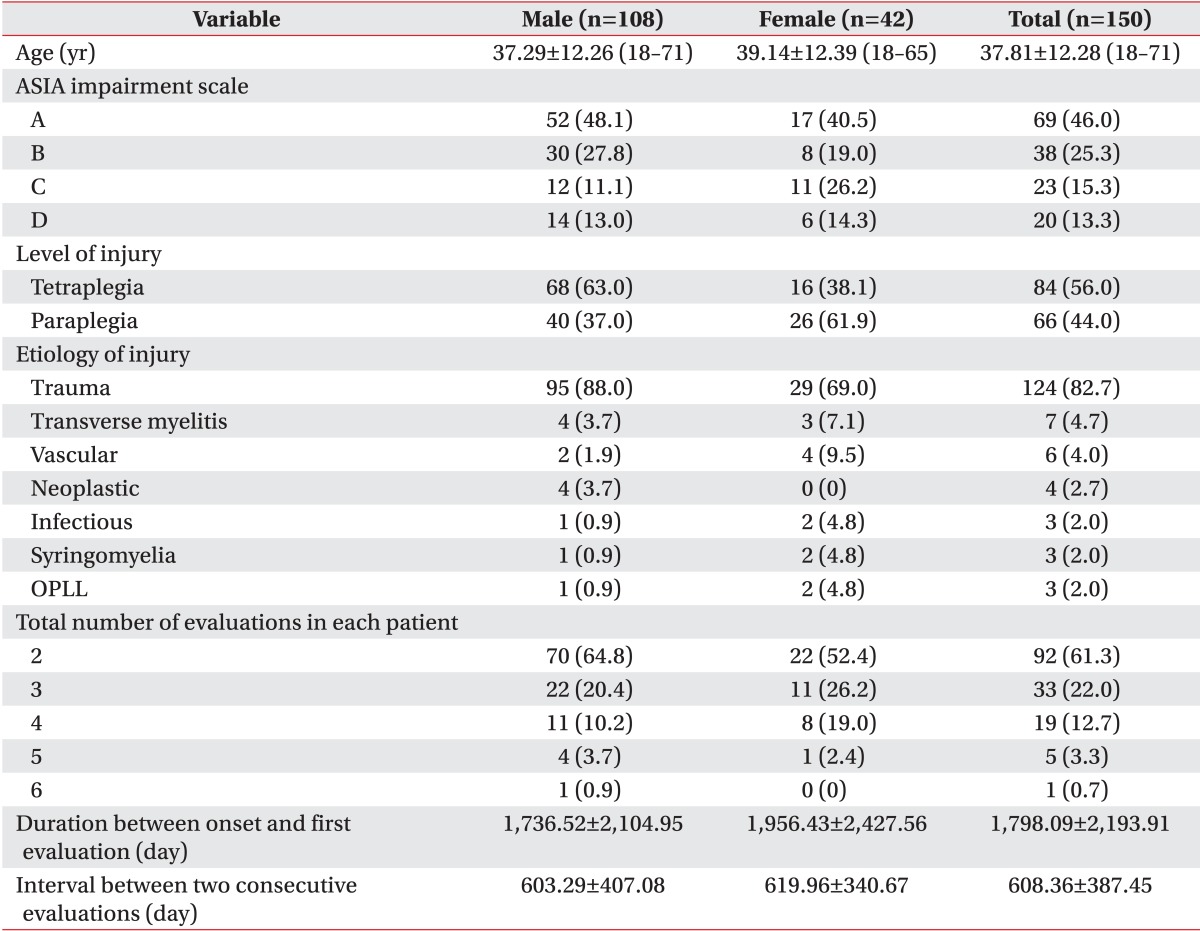
Demographic data of the study subjects enrolled in this study and general characteristics of evaluations are summed up in Table 1. The age and neurologic status in Table 1 are based on the data collected at the time of the first evaluation during the study period. The etiology of SCI in the vast majority of study subjects was trauma. The total number of evaluations each study subject had undergone varied between two and six, which added up to a total number of 390 evaluations.
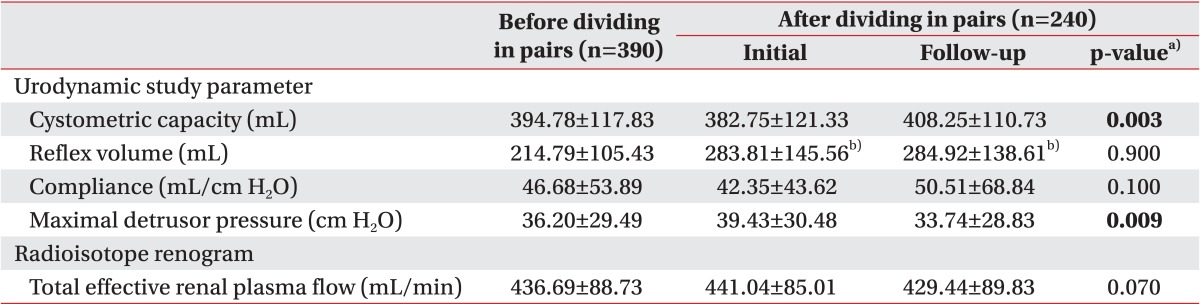
UDS parameters and ERPF before and after dividing into pairs are shown in Table 2. The mean value of reflex volume before dividing into pairs included 280 evaluations, which showed involuntary detrusor contraction.
The direct comparison of initial and follow-up results showed a significant increase in cystometric capacity and a significant decrease in maximal detrusor pressure on follow-up compared to the initial evaluation, which was possibly due to the treatment based on the result of the initial evaluation. Reflex volume, compliance, and total ERPF did not show any significant differences.
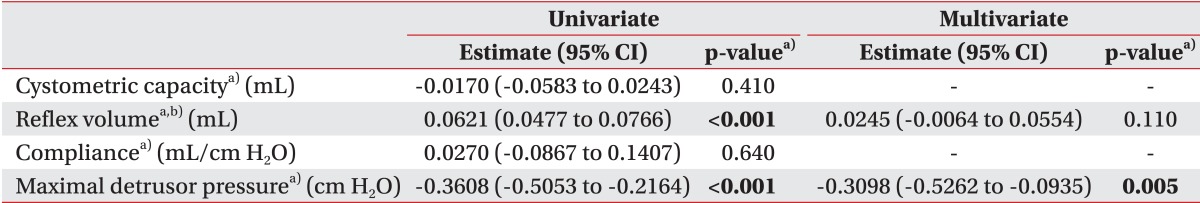
On univariate analysis, reflex volume and maximal detrusor pressure showed statistical significance. Multivariate analysis was then performed with reflex volume and maximal detrusor pressure, which showed statistical significance for maximal detrusor pressure with a negative odds ratio (Table 3).
Multicollinearity between reflex volume and maximal detrusor pressure was analyzed, which showed a variance inflation factor of 1.2.
Go to : 
Upper urinary tract deterioration is affected by various factors besides neurogenic bladder status, which also includes voiding method [19] amongst others. Therefore it is rather difficult to demonstrate a valid independent association between a single UDS parameter and upper urinary tract deterioration. The fact that most UDS parameters cannot be assumed to be independent from one another aggravates the level of difficulty.
To account for these obstacles, we first analyzed the change of UDS parameters with the change of renal function, instead of analyzing it with morphological change of the upper urinary system. We expected an increase of sensitivity to find any significant associations. Second, we performed statistical analysis by mixed-model regression techniques to account for clustered data, in order to correct for any interdependency between UDS parameters.
So far, several previous studies have demonstrated the association between UDS parameters and upper urinary tract complications. In the most prominent study among them, intravesical leak point pressure greater than 40 cm H2O was associated with upper urinary tract complications [8]. Other studies reported the following parameters as potential risk factor for upper urinary tract complication: detrusor pressure greater than 6 cm H2O that persists for a period of longer than 10 seconds [9]; low compliance which in one study was defined as at or below 20 mL/cm H2O [10], and in another study as below 12.5 mL/cm H2O [14]; MUPG greater than 80 cm H2O [11]; detrusor pressure greater than 40 cm H2O [12]; and duration of uninhibited bladder contraction [13].
In this study, a significant inverse relationship between renal function and maximal detrusor pressure was observed. An increase of maximal detrusor pressure is likely to result in decline of renal function and vice versa. We did not find any significant association between compliance and renal function.
In our study, we did not define any specific values as 'normal' as there is a lack of established range of normal values in UDS parameters [20]. We merely analyzed any impact by change of values, and found out that change of maximal detrusor pressure may help to predict change of renal function in SCI patients. Thus, the results of this study may not suffice as evidence, when interpreting the results of a single UDS evaluation. However, they are the best so far, when interpreting results of consecutive UDS evaluations.
Thus, we can draw two conclusions from the results of this study. First, among the four UDS parameters investigated in this study, maximal detrusor pressure should be monitored most closely, when managing neurogenic detrusor overactivity in SCI patients. This study does not exclude the existence of any other parameters with a significant association to renal function. However, so far it deserves the most attention.
Second, UDS is recommended as the gold standard for the evaluation of urinary tract dysfunction in SCI patients [6], and regular follow-up of UDS is warranted for protection of the upper urinary tract and maintenance of continence [7]; however, it is not often used in practice [21,22,23]. The European Association of Urology recommends in neurogenic lower urinary tract dysfunction, a follow-up UDS every two years in patients without detrusor overactivity and with normal bladder compliance, and at least once per year in patients with detrusor overactivity and/or low bladder compliance [24]. On the other hand, a proposed guideline by the 'SCI Think Tank' in the UK recommends a baseline UDS between three and six months after SCI, and follow-up UDS only in high-risk patients [25]. This study not only shows a significant association between UDS parameters and renal function, it also shows that change in UDS parameters in a patient can indicate change in renal function. This, again, shows that UDS in SCI patients is essential not just as a baseline evaluation, but also as a follow-up assessment. Reference on the desirable interval of UDS is still lacking [26], and it demands for further research in the future.
Go to : 
References
1. McGuire EJ, Savastano J. Comparative urological outcome in women with spinal cord injury. J Urol. 1986; 135:730–731. PMID: 3959193.

2. Gerridzen RG, Thijssen AM, Dehoux E. Risk factors for upper tract deterioration in chronic spinal cord injury patients. J Urol. 1992; 147:416–418. PMID: 1732606.

3. Webb DR, Fitzpatrick JM, O'Flynn JD. A 15-year follow-up of 406 consecutive spinal cord injuries. Br J Urol. 1984; 56:614–617. PMID: 6534476.

4. Viera A, Merritt JL, Erickson RP. Renal function in spinal cord injury: a preliminary report. Arch Phys Med Rehabil. 1986; 67:257–259. PMID: 3964061.
5. Sauerwein D. Surgical treatment of spastic bladder paralysis in paraplegic patients: sacral deafferentation with implantation of a sacral anterior root stimulator. Urologe A. 1990; 29:196–203. PMID: 2399626.
6. Watanabe T, Rivas DA, Chancellor MB. Urodynamics of spinal cord injury. Urol Clin North Am. 1996; 23:459–473. PMID: 8701559.

7. Nosseir M, Hinkel A, Pannek J. Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol Urodyn. 2007; 26:228–233. PMID: 16998859.

8. McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981; 126:205–209. PMID: 7196460.

9. McGuire EJ, Savastano JA. Urodynamics and management of the neuropathic bladder in spinal cord injury patients. J Am Paraplegia Soc. 1985; 8:28–32. PMID: 3842979.

10. Hackler RH, Hall MK, Zampieri TA. Bladder hypocompliance in the spinal cord injury population. J Urol. 1989; 141:1390–1393. PMID: 2724437.

11. Killorin W, Gray M, Bennett JK, Green BG. The value of urodynamics and bladder management in predicting upper urinary tract complications in male spinal cord injury patients. Paraplegia. 1992; 30:437–441. PMID: 1635794.

12. Shingleton WB, Bodner DR. The development of urologic complications in relationship to bladder pressure in spinal cord injured patients. J Am Paraplegia Soc. 1993; 16:14–17. PMID: 8426179.

13. Linsenmeyer TA, Bagaria SP, Gendron B. The impact of urodynamic parameters on the upper tracts of spinal cord injured men who void reflexly. J Spinal Cord Med. 1998; 21:15–20. PMID: 9541882.

14. Weld KJ, Graney MJ, Dmochowski RR. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. J Urol. 2000; 163:1228–1233. PMID: 10737503.

16. Bih LI, Changlai SP, Ho CC, Lee SP. Application of radioisotope renography with technetium-99m mercaptoacetyltriglycine on patients with spinal cord injuries. Arch Phys Med Rehabil. 1994; 75:982–986. PMID: 8085934.

17. Price CP, Finney H. Developments in the assessment of glomerular filtration rate. Clin Chim Acta. 2000; 297:55–66. PMID: 10841908.

18. Jenkins MA, Brown DJ, Ierino FL, Ratnaike SI. Cystatin C for estimation of glomerular filtration rate in patients with spinal cord injury. Ann Clin Biochem. 2003; 40(Pt 4):364–368. PMID: 12880536.
19. Weld KJ, Dmochowski RR. Effect of bladder management on urological complications in spinal cord injured patients. J Urol. 2000; 163:768–772. PMID: 10687973.

20. Mahfouz W, Al Afraa T, Campeau L, Corcos J. Normal urodynamic parameters in women. Part II. Invasive urodynamics. Int Urogynecol J. 2012; 23:269–277. PMID: 22011933.
21. Razdan S, Leboeuf L, Meinbach DS, Weinstein D, Gousse AE. Current practice patterns in the urologic surveillance and management of patients with spinal cord injury. Urology. 2003; 61:893–896. PMID: 12735998.

22. Bycroft J, Hamid R, Bywater H, Patki P, Craggs M, Shah J. Variation in urological practice amongst spinal injuries units in the UK and Eire. Neurourol Urodyn. 2004; 23:252–256. PMID: 15098222.

23. Al Taweel W, Alkhayal A. Neurogenic bladder evaluation and management after spinal cord injury: Current practice among urologists working in Saudi Arabia. Urol Ann. 2011; 3:24–28. PMID: 21346829.

24. Stohrer M, Blok B, Castro-Diaz D, Chartier-Kastler E, Del Popolo G, Kramer G, et al. EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol. 2009; 56:81–88. PMID: 19403235.
25. Abrams P, Agarwal M, Drake M, El-Masri W, Fulford S, Reid S, et al. A proposed guideline for the urological management of patients with spinal cord injury. BJU Int. 2008; 101:989–994. PMID: 18279449.

26. Cameron AP, Rodriguez GM, Schomer KG. Systematic review of urological followup after spinal cord injury. J Urol. 2012; 187:391–397. PMID: 22177149.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download