Abstract
Objective
To characterize neuropathic pain in patients with spinal cord injury (SCI) according to classification used in the study by Baron et al. (Baron classification), a classification of neuropathic pain based on the mechanism. To also compare the patterns of neuropathic pain in SCI patients with those in patients with other etiologies and to determine the differences in patterns of neuropathic pain between the etiologies.
Methods
This was a descriptive cross-sectional study. We used the Baron classification to investigate the characteristics of neuropathic pain in SCI. Sixty-one SCI patients with neuropathic pain (The Leeds assessment of neuropathic symptoms and signs score ≥12) were enrolled in this study between November 2012 and August 2013, after excluding patients <20 of age, patients with visual analog scale (VAS) score <3, pregnant patients, and patients with systemic disease or pain other than neuropathic pain.
Results
The most common pain characteristic was pricking pain followed by electrical pain and numbness. The mean VAS score of at-level neuropathic pain was 7.51 and that of below-level neuropathic pain was 6.83. All of the patients suffered from rest pain, but 18 (54.6%) patients with at-level neuropathic pain and 20 (50.0%) patients with below-level neuropathic pain suffered from evoked pain. There was no significant difference in between at-level and below-level neuropathic pains.
Conclusion
The result was quite different from the characteristics of post-herpetic neuralgia, but it was similar to the characteristics of diabetic neuropathy as shown in the study by Baron et al., which means that sensory nerve deafferentation may be the most common pathophysiologic mechanism of neuropathic pain after SCI. Since in our study, we included short and discrete symptoms and signs based on diverse mechanisms, our results could be helpful for determining further evaluation and treatment.
Spinal cord injury (SCI) causes impairments in motor function and decreased sensory function along with many other complications. One of the major complications is chronic pain [1,2]. Not only is chronic pain disabling by itself, but it also interferes with the patient's participation in rehabilitation and leads to other complications, such as cognitive dysfunction, emotional changes, and overall poor quality of life [2,3,4]. According to previous studies, an average of about two-thirds of people with SCI report some form of pain and nearly one-third rate their pain as severe [5]. Recent studies reported that the prevalence of pain was as high as 77% to 86% [5,6,7]. The International Association for the Study of Pain broadly classified pain following SCI into nociceptive and neuropathic pain, and defined neuropathic pain as pain caused by a lesion or disease of the somatosensory nervous system [8]. Neuropathic pain is caused by dysfunctions in the peripheral or central nervous system without peripheral nociceptive stimulation, and the clinical features of neuropathic pain are similar regardless of the underlying causes [9,10].
Therefore, many studies have been conducted for the treatment of neuropathic pain, but studies that provide a validated rationale and information on the drugs having an effect on specific pain characteristics are lacking. The main cause for this occurrence is thought to be the lack of consensus on the selection of specific pain characteristics and its underlying mechanism, as well as the lack of a specific definition of the primary outcome. To overcome this problem, Baron et al. [11] studied patients with diabetic polyneuropathy and post-herpetic neuralgia, and classified neuropathic pain into five clusters according to the distribution of pain characteristics; the findings were associated with the mechanism of pain. Using this classification, we can identify the most appropriate patient subgroup that would respond optimally to a specific medication. Specific symptom profiles of patients with neuropathic pain are required for performing meaningful clinical research and patient selection.
Many clinical studies on neuropathic pain after SCI report the pain frequency and characteristics, but studies on pain characteristics based on the mechanism are rare, especially using the classification put forward by Baron et al. [11]. The first aim of the present study was to describe and evaluate the characteristics of neuropathic pain in patients with SCI. The second aim was to compare the patterns of neuropathic pain in SCI patients with those in patients with other etiologies, and to determine the differences in patterns of neuropathic pain between etiologies.
This was a descriptive cross-sectional study. The survey was performed between November 2012 and August 2013. Sixty-one SCI patients who were suffering from at-level neuropathic pain and/or below-level neuropathic pain were enrolled in this study. Since we evaluated at-level neuropathic pain and below-level neuropathic pain separately, the total data set used for the analysis included 73 assessments. We used the Leeds assessment of neuropathic symptoms and signs (LANSS) score [12] to confirm neuropathic pain, and patients with a LANSS score of 12 and above were enrolled in the study. Exclusion criteria were the patients with a visual analog scale (VAS) pain score lower than 3, pregnant patients, patients with systemic disease, and patients less than 20 years of age. Patients suffering from pain other than neuropathic pain were excluded to avoid interference from other pain types. Therefore, patients with urinary infection, heterotopic ossification, pressure ulcers, and severe spasticity (Ashworth score of 3 or more), i.e., patients having severe pain that may alter the severity and characteristics of neuropathic pain, were also excluded [6].
All of the patients were examined and classified according to the International Standards for Neurological Classification of Spinal Cord Injury (2011). We recorded the demographic and clinical characteristics of each patient. All of the patients with neuropathic pain were assessed for the initial pain characteristic and intensity using the VAS after discontinuation of prior medication for 3 days. We used the same classification as that used in the study by Baron et al. [11], in which the patients were divided according to the characteristics of neuropathic pain, to evaluate the symptoms and signs of neuropathic pain in SCI patients. The symptoms and signs of neuropathic pain were divided into rest pain and evoked pain. Rest pain included spontaneous electric shock-like pain, burning pain, pricking pain, and numbness. Evoked pain included allodynia, heat hyperalgesia, and pressure hyperalgesia. After categorizing neuropathic pain, we evaluated the severity of pain using the VAS score. Also, we subdivided neuropathic pain into 'at-level' neuropathic pain and 'below-level' neuropathic pain [13]. At-level neuropathic pain is the neuropathic pain that occurs at the neurological level of injury and two segments above and below the neurological level of injury, and it can be further divided into radicular or central pain. This pain is usually thought to be due to nerve root pathology (radicular) or changes within the spinal cord or possibly supraspinal structures (central). Below-level neuropathic pain is present at least three segments below the neurological level of injury and is due to lesions in the spinothalamocortical pathways [14]. The distribution of neuropathic pain in SCI patients was compared to that of neuropathic pain in patients with diabetes and post-herpetic neuralgia, as described in the study by Baron et al. [11].
Statistical analyses were conducted using SPSS ver. 19.0 (IBM, Armonk, New York, USA). For the comparison of differences between complete and incomplete SCI patients, independent t-tests and chi-square tests were used. The results are presented as mean±standard deviation or percent. The p-values of <0.05 were considered statistically significant.
The demographic and clinical characteristics of the patients are listed in Table 1. The average patient age was 50 years and the number of males was 24 (39.3%). There were 44 chronic SCI patients (72.1%) and the neurological level of injury was cervical in 25 patients, thoracic in 30 patients, lumbar in 5 patients, and sacral in 1 patient. The number of patients with complete injury (American Spinal Injury Association [ASIA] A) was 16 (26.2%), and the number of patients with incomplete injury (ASIA B, C, D) was 45 (73.8%). Of the 61 patients, 33 patients (54.1%) suffered from at-level neuropathic pain, 40 patients (65.6%) suffered from below-level neuropathic pain, and 12 patients (19.7%) suffered from both at-level and below-level neuropathic pains.
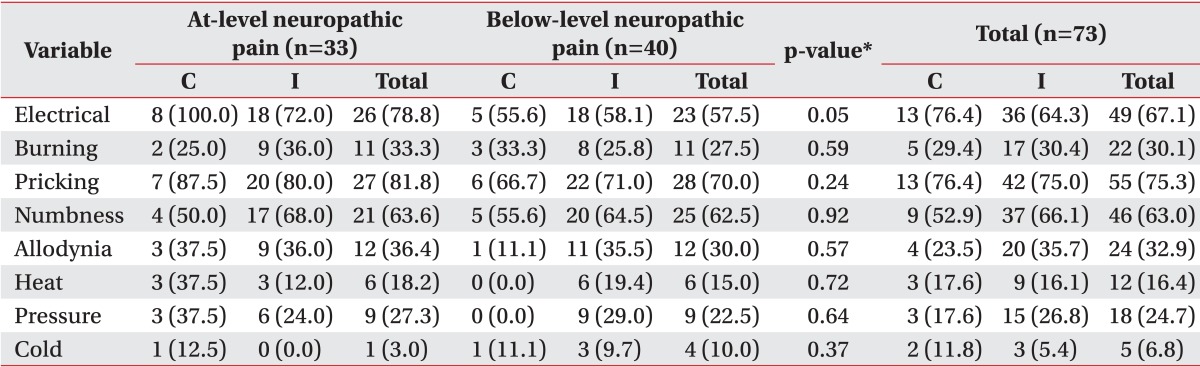
The mean VAS scores of at-level neuropathic pain ranged from 6.31 (numbness) to 10.00 (cold hyperalgesia), and the mean VAS scores of below-level neuropathic pain ranged from 5.58 (heat) to 7.75 (cold hyperalgesia). Among the characteristics of neuropathic pain, pricking (27 patients, 81.8%) was the most common pain characteristic in patients with at-level neuropathic pain, followed by electrical pain (26 patients, 78.8%) and numbness (21 patients, 63.6%). Also, the most common pain characteristic described by patients with below-level neuropathic pain was pricking (28 patients, 70.0%), followed by numbness (25 patients, 62.5%) and electrical pain (23 patients, 57.5%) (Table 2).
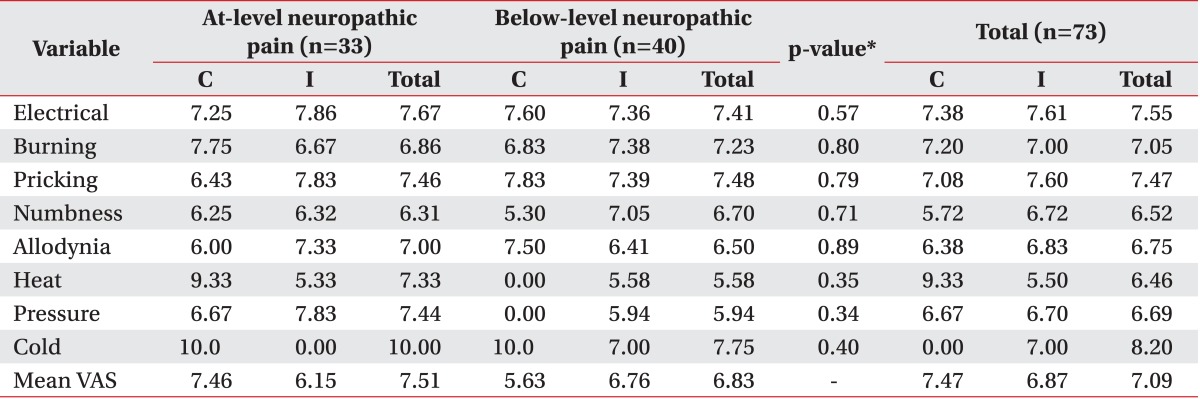
The mean VAS score of at-level neuropathic pain was 7.51 and the mean VAS score of below-level neuropathic pain was 6.83. No significant difference was observed in the pain characteristics between the two groups (Table 3; Figs. 1, 2).
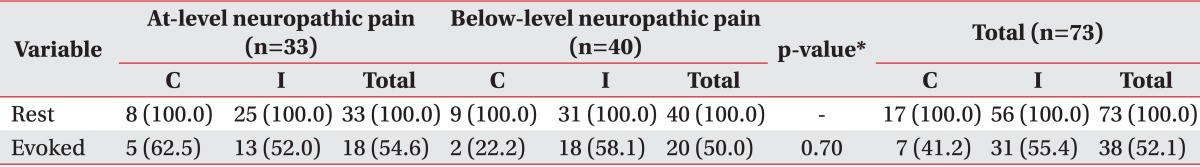
When we grouped the prevalence of pain according to pain characteristics, all of the patients enrolled in this study suffered from rest pain (100.0%), whereas 18 patients (54.6%) with at-level neuropathic pain and 20 patients (50.0%) with below-level neuropathic pain suffered from evoked pain. No significant differences were observed in the prevalence of rest pain and evoked pain between the two groups (Table 4, Fig. 3).
There have been many studies on the prevalence and features of neuropathic pain after SCI, since neuropathic pain is common. The two widely used classifications in rehabilitation medicine are Bryce/Ragnarsson spinal cord injury pain taxonomy and International Spinal Cord Injury Pain Classification, which categorize pain based on the etiology and location [13,15,16]. However, some studies demonstrate that for achieving a better treatment outcome, it would be more appropriate to use the mechanism-based classification rather than the etiology-based classification [17,18]. Unfortunately, there have been few studies that systemically describe the characteristics of neuropathic pain based on the mechanism. Only recently, the characteristics of neuropathic pain based on the mechanism were described in a study by Baron et al. [11]. Although the researchers focused on neuropathic pain associated with diabetes and post-herpetic neuralgia, the classification was based on the underlying mechanism of neuropathic pain and could be applied to SCI patients as well. After peripheral nerve injury, new channel formation, up-regulation, down-regulation, and new expression of receptors can occur. This change is similar to the phenomenon which occurs after spinal cord and brain injury [17]. Baron classification used symptom profiles in the painDETECT questionnaire and classified them into 5 clusters. The painDETECT questionnaire was based on the characteristic clinical neuropathic symptoms [19], and there are concerns that the individual pattern of sensory symptoms can be closely related to the mechanisms of pain [11,20,21]. It is considered that the mechanism of evoked pain-thermal (heat and cold), pressure, and allodynia-is related with central sensitization. With respect to rest pain, attack (electrical) and pricking pain symptoms are known to be related with ectopic or ephaptic discharges that are spontaneously generated by all types of fibers due to the processes like damaged Na+ channel (leaking) or overactivated Na+ channels. On the other hand, the spontaneous activity of unmyelinated fibers mainly triggers burning and numbness. However, further studies are required [21,22,23]. The present study used the Baron classification [11] to evaluate the characteristics of neuropathic pain after SCI.
We found that there was no difference in the prevalence of at-level neuropathic pain and below-level neuropathic pain, and the most common pain characteristic was pricking pain (in 81.8% of patients with at-level neuropathic pain and in 70.0% of patients with below-level neuropathic pain). The next most common pain characteristics were electrical pain (in 78.8% of patients with in at-level neuropathic pain and in 57.5% of patients with below-level neuropathic pain) and numbness (in 63.6% of patients with at-level neuropathic pain and in 62.5% of patients with below-level neuropathic pain). This result was quite different from the characteristics of previous studies on post-herpetic neuralgia. Out of the seven pain characteristics, prickling pain was the fifth most frequent (38%), while numbness was the least frequent (14%). However, it was similar to that in diabetic neuropathy; out of the seven pain characteristics, prickling pain was the most frequent (35%), while numbness was the third most frequent (30%) as described by Baron et al. [11]. This means that sensory nerve deafferentation may be the most common pathophysiologic mechanism of neuropathic pain after SCI. However, this result should be interpreted carefully due to the limited number of participants and the lack of cluster analysis. Furthermore, same symptoms can develop due to two or more mechanisms, and also in one disease, spectrum of diverse mechanisms influences each individual differently [17]. In previous studies, the most frequently used words to describe neuropathic pain were throbbing, tiring, hot and tingling, electric shock, pins and needles, burning, numb, hot, pressure, squeezing, and shooting; the average VAS scores ranged from 5.16 to 6.38 according to the time after injury [6,22,24]. However, these characteristics of neuropathic pain were diverse and were not based on a mechanistic classification. The results of the present study show the diverse nature of neuropathic pain in SCI patients, but they may not be helpful in further studies including symptom- and sign-based treatments. Since we included short and discrete symptoms and signs based on diverse mechanisms in our study, our results could be helpful for determining further evaluation and treatment.
We also subgrouped the pain characteristics into two subgroups, i.e., rest pain and evoked pain. Each patient complained of rest pain, but about 50% of patients complained of evoked pain. Based on these results, we think that drugs that interfere with sensory nerve deafferentation would be the most suitable for the treatment of neuropathic pain after SCI, although further studies are required because many patients reported different pain characteristics. There have been many studies on the treatment of neuropathic pain, and many drugs, such as nonsteroidal anti-inflammatory drugs, opiates, antidepressants, antiepileptics, etc., have been found to be effective in treating neuropathic pain. Gabapentin was found to be effective in clinical trials for diabetic neuropathy, post-herpetic neuralgia, neuropathic pain after SCI, cancer, and other neuropathic pain [25,26,27,28]. Pregabalin, an antiepileptic drug similar to gabapentin, was found to be effective in studies on diabetic neuropathy, post-herpetic neuralgia, and neuropathic pain after SCI [29,30,31,32]. Other drugs, such as topical lidocaine, venlafaxine, and bupropion, have also been found to be effective in treating neuropathic pain [33,34,35]. However, cross-sectional studies have revealed that patients with neuropathic pain continue to experience moderate pain even while taking the prescribed medications [36,37,38]. Also, these studies did not analyze subgroups of patients with similar clinical phenomena and described the effect of drugs by comparing the general responses to the drugs rather than by investigating the mechanism of pain. Finally, there is a lack of information on how drugs affect the specific symptoms and signs of pain [39]. A more systematic and effective approach would be to develop treatment guidelines for the effects of drugs based on symptoms.
A limitation of the current study is that only 61 SCI patients were included. Therefore, we could not perform a cluster analysis as that performed in the study by Baron et al. [11].
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (No. NRF-2013R1A1A1004622).
References
1. Budh CN, Osteraker AL. Life satisfaction in individuals with a spinal cord injury and pain. Clin Rehabil. 2007; 21:89–96. PMID: 17213246.

2. Burchiel KJ, Hsu FP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine (Phila Pa 1976). 2001; 26(24 Suppl):S146–S160. PMID: 11805622.
3. Westgren N, Levi R. Quality of life and traumatic spinal cord injury. Arch Phys Med Rehabil. 1998; 79:1433–1439. PMID: 9821906.

4. Wollaars MM, Post MW, van Asbeck FW, Brand N. Spinal cord injury pain: the influence of psychologic factors and impact on quality of life. Clin J Pain. 2007; 23:383–391. PMID: 17515736.

5. Stormer S, Gerner HJ, Gruninger W, Metzmacher K, Follinger S, Wienke C, et al. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord. 1997; 35:446–455. PMID: 9232750.

6. Celik EC, Erhan B, Lakse E. The clinical characteristics of neuropathic pain in patients with spinal cord injury. Spinal Cord. 2012; 50:585–589. PMID: 22430511.

7. Fenollosa P, Pallares J, Cervera J, Pelegrin F, Inigo V, Giner M, et al. Chronic pain in the spinal cord injured: statistical approach and pharmacological treatment. Paraplegia. 1993; 31:722–729. PMID: 7507585.

8. Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008; 70:1630–1635. PMID: 18003941.

9. Ro LS, Chang KH. Neuropathic pain: mechanisms and treatments. Chang Gung Med J. 2005; 28:597–605. PMID: 16323550.
10. Widerstrom-Noga EG, Turk DC. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: influence of pain and psychosocial characteristics. Spinal Cord. 2003; 41:600–609. PMID: 14569261.

11. Baron R, Tolle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: differences in demographic data and sensory symptoms. Pain. 2009; 146:34–40. PMID: 19592166.

12. Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001; 92:147–157. PMID: 11323136.

13. Bryce TN, Ragnarsson KT. Pain after spinal cord injury. Phys Med Rehabil Clin N Am. 2000; 11:157–168. PMID: 10680163.

14. Siddall PJ, Taylor DA, Cousins MJ. Classification of pain following spinal cord injury. Spinal Cord. 1997; 35:69–75. PMID: 9044512.

15. Bryce TN, Budh CN, Cardenas DD, Dijkers M, Felix ER, Finnerup NB, et al. Pain after spinal cord injury: an evidence-based review for clinical practice and research: report of the National Institute on Disability and Rehabilitation Research Spinal Cord Injury Measures meeting. J Spinal Cord Med. 2007; 30:421–440. PMID: 18092558.
16. Bryce TN, Dijkers MP, Ragnarsson KT, Stein AB, Chen B. Reliability of the Bryce/Ragnarsson spinal cord injury pain taxonomy. J Spinal Cord Med. 2006; 29:118–132. PMID: 16739555.

17. Finnerup NB, Jensen TS. Mechanisms of disease: mechanism-based classification of neuropathic paina critical analysis. Nat Clin Pract Neurol. 2006; 2:107–115. PMID: 16932532.

18. Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, et al. Towards a mechanism-based classification of pain? Pain. 1998; 77:227–229. PMID: 9808347.

19. Freynhagen R, Baron R, Gockel U, Tolle TR. painDE TECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006; 22:1911–1920. PMID: 17022849.
20. Baron R. Mechanisms of disease: neuropathic pain: a clinical perspective. Nat Clin Pract Neurol. 2006; 2:95–106. PMID: 16932531.
21. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010; 9:807–819. PMID: 20650402.

22. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999; 353:1959–1964. PMID: 10371588.

23. Harden RN. Chronic neuropathic pain: mechanisms, diagnosis, and treatment. Neurologist. 2005; 11:111–122. PMID: 15733333.
24. Calmels P, Mick G, Perrouin-Verbe B, Ventura M. French Society for Physical Medicine and Rehabilitation. Neuropathic pain in spinal cord injury: identification, classification, evaluation. Ann Phys Rehabil Med. 2009; 52:83–102. PMID: 19909700.

25. Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003; 25:81–104. PMID: 12637113.

26. Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998; 280:1831–1836. PMID: 9846777.

27. Rice AS, Maton S. Postherpetic Neuralgia Study Group. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001; 94:215–224. PMID: 11690735.

28. Caraceni A, Zecca E, Martini C, De Conno F. Gabapentin as an adjuvant to opioid analgesia for neuropathic cancer pain. J Pain Symptom Manage. 1999; 17:441–445. PMID: 10388250.

29. Dworkin RH, Corbin AE, Young JP Jr, Sharma U, LaMoreaux L, Bockbrader H, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003; 60:1274–1283. PMID: 12707429.

30. Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005; 115:254–263. PMID: 15911152.

31. Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004; 110:628–638. PMID: 15288403.

32. Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006; 67:1792–1800. PMID: 17130411.

33. Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996; 65:39–44. PMID: 8826488.

34. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004; 110:697–706. PMID: 15288411.

35. Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology. 2001; 57:1583–1588. PMID: 11706096.

36. McDermott AM, Toelle TR, Rowbotham DJ, Schaefer CP, Dukes EM. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain. 2006; 10:127–135. PMID: 16310716.

37. O'Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009; 27:95–112. PMID: 19254044.
38. Tolle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications. 2006; 20:26–33. PMID: 16389164.
39. O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009; 122(10 Suppl):S22–S32. PMID: 19801049.
Fig. 1
Prevalence and average visual analog scale (VAS) score (rest pain). Among the characteristics of neuropathic pain, pricking was the most common pain characteristic in patients with at-level neuropathic pain and below-level neuropathic pain, followed by electrical pain and numbness. The mean VAS score of at-level neuropathic pain was 6.89 and that of below-level neuropathic pain was 7.79. There was no significant difference in the prevalence and VAS score of neuropathic pain between the two groups.
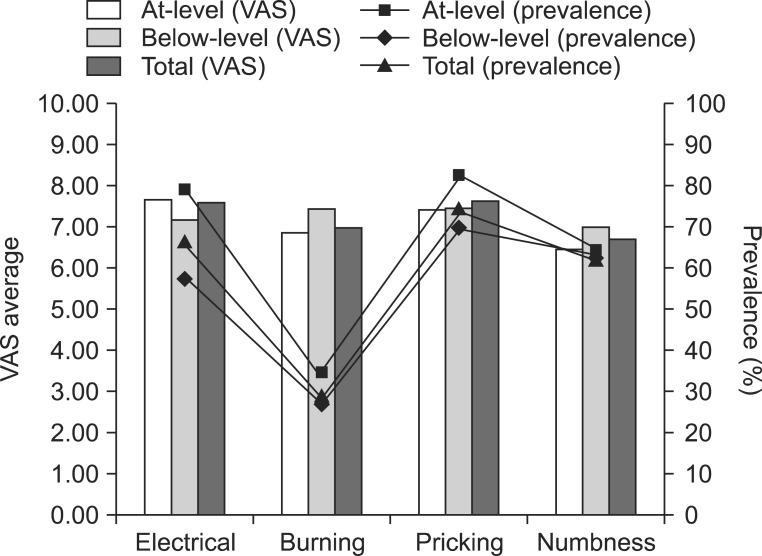
Fig. 2
Prevalence and average visual analog scale (VAS) score (evoked pain). Among the characteristics of neuropathic pain, pricking was the most common pain characteristic in patients with at-level neuropathic pain and below-level neuropathic pain, followed by electrical pain and numbness. The mean VAS score of at-level neuropathic pain was 7.28 and that of below-level neuropathic pain was 6.54. There was no significant difference in the prevalence and VAS score of neuropathic pain between the two groups.
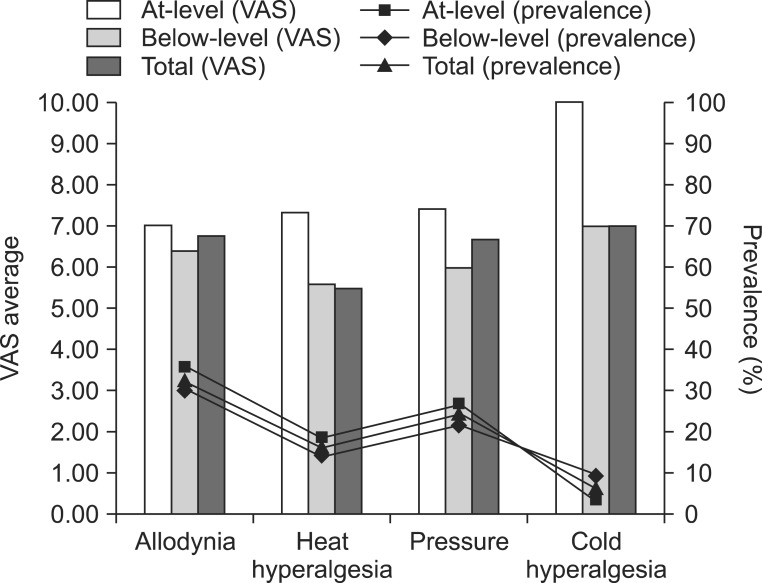
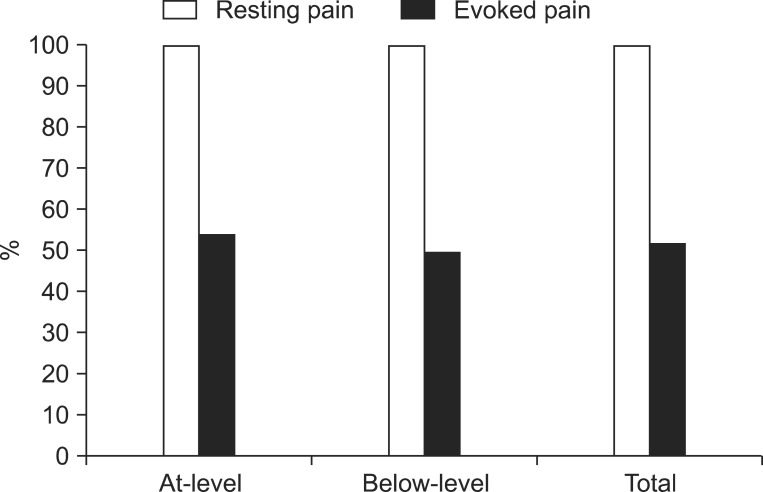
Fig. 3
Prevalence of rest pain and evoked pain. All of the patients enrolled in this study suffered from rest pain, whereas about half of the patients suffered from evoked pain. No significant differences were observed in the prevalence of rest pain and evoked pain between the two groups.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download