This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
To investigate the effects of simultaneous, bihemispheric, dual-mode stimulation using repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) on motor functions and cortical excitability in healthy individuals.
Methods
Twenty-five healthy, right-handed volunteers (10 men, 15 women; mean age, 25.5 years) were enrolled. All participants received four randomly arranged, dual-mode, simultaneous stimulations under the following conditions: condition 1, high-frequency rTMS over the right primary motor cortex (M1) and sham tDCS over the left M1; condition 2, high-frequency rTMS over the right M1 and anodal tDCS over the left M1; condition 3, high-frequency rTMS over the right M1 and cathodal tDCS over the left M1; and condition 4, sham rTMS and sham tDCS. The cortical excitability of the right M1 and motor functions of the left hand were assessed before and after each simulation.
Results
Motor evoked potential (MEP) amplitudes after stimulation were significantly higher than before stimulation, under the conditions 1 and 2. The MEP amplitude in condition 2 was higher than both conditions 3 and 4, while the MEP amplitude in condition 1 was higher than condition 4. The results of the Purdue Pegboard test and the box and block test showed significant improvement in conditions 1 and 2 after stimulation.
Conclusion
Simultaneous stimulation by anodal tDCS over the left M1 with high-frequency rTMS over the right M1 could produce interhemispheric modulation and homeostatic plasticity, which resulted in modulation of cortical excitability and motor functions.
Go to :

Keywords: Bihemispheric stimulation, Transcranial direct current stimulation (tDCS), Transcranial magnetic stimulation (TMS), Interhemispheric modulation, Motor function
INTRODUCTION
The noninvasive brain stimulation methods, such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), are currently used for modulation of neural excitability [
1,
2]. The application of high-frequency rTMS over the primary motor cortex (M1) has been shown to increase the cortical excitability during or after the stimulation in healthy subjects [
3] and stroke patients [
4]. Anodal tDCS over M1 has been shown to increase corticomotor excitability and improve contralateral hand function [
5,
6]. Conversely, cathodal tDCS over the dominant M1 could decrease cortical excitability and improve functions of the non-dominant hands in healthy subjects [
7,
8,
9]. A previous research suggested that it was the interhemispheric interaction that modulated the inhibition of transcallosal connections from the dominant motor cortex [
10,
11]. The recent studies have demonstrated that the simultaneous bilateral tDCS over both M1s was more effective stimulation than the unilateral tDCS [
9,
12,
13].
Until now, no research has been published on the effects of bihemispheric, dual-mode stimulation using both tDCS and rTMS. In this study, we investigated the interactive effects of simultaneous, dual-mode, noninvasive brain stimulation on corticomotor excitability and motor functions. We hypothesized that simultaneously stimulating both motor cortices with high-frequency rTMS over the non-dominant hemisphere and tDCS over the dominant hemisphere, might induce the add-on effect of unihemispheric stimulation through interhemispheric modulation. Therefore, the objective of this study was to investigate whether simultaneous tDCS over the contralateral (dominant) M1 would modulate the effects of high-frequency rTMS over the target (non-dominant) M1, and to determine their effects on the motor function and corticomotor excitability in healthy subjects.
Go to :

MATERIALS AND METHODS
Twenty-five healthy volunteers (10 men, 15 women; mean age, 25.5±2.1 years; range, 21-27 years) participated in the experiment. The exclusion criteria were as follows: 1) any clinically significant or unstable medical disorder, 2) any neuropsychiatric problem, 3) any history of epilepsy, and 4) participation in another ongoing study. The experiments were conducted with the understanding and written consent of each participant, and ethical approval was provided by the Institutional Review Board. All participants were right-handed with laterality quotients greater than 80, according to the Edinburgh Handedness Inventory.
This study was designed as a double-blind, random-order crossover trial. The participants underwent four randomly arranged, dual-mode stimulations under the following conditions: 1) condition 1, 10 Hz rTMS over the target M1 (non-dominant, right hemisphere) and sham tDCS over the contralateral M1 (dominant, left hemisphere); 2) condition 2, 10 Hz rTMS over the target M1 and anodal tDCS over the contralateral M1; 3) condition 3, 10 Hz rTMS over the target M1 and cathodal tDCS over the contralateral M1; and 4) condition 4, sham rTMS over the target M1 and sham tDCS over the contralateral M1. Each stimulation session was conducted on a different day, such that the consecutive stimulation sessions were separated by a washout period of at least 24 hours (
Fig. 1). The magnitude of change in corticomotor excitability in the right M1 was measured by amplitude and latency of motor evoked potential (MEP). For the assessment of hand motor function, participants performed the Purdue Pegboard test, the box and block test, and the grip strength test with their left hands prior to and immediately after the stimulation in each condition.
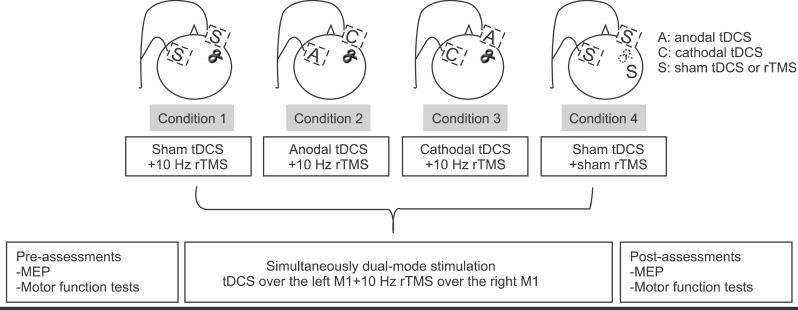 | Fig. 1Experimental design. Condition 1, high-frequency rTMS over the right M1 and sham tDCS over the left M1; condition 2, high-frequency rTMS over the right M1 and simultaneous anodal tDCS over the left M1; condition 3, high-frequency rTMS over the right M1 and simultaneous cathodal tDCS over the left M1; and condition 4, sham tDCS over the left M1 and sham rTMS over the right M1. Hand motor function tests, MEP amplitudes, and MEP latency were assessed immediately before and after the stimulation in each condition. rTMS, transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; M1, primary motor cortex; MEP, motor evoked potential. 
|
Determination of motor cortex and resting motor thresholds
To determine the optimal scalp location for bilateral M1s and the location for the intensity of rTMS and to evaluate the cortical excitability, a single-pulse TMS was performed on each subject prior to each session. The subjects were comfortably seated in a reclining armchair with both hands pronated on a pillow. Electromyography (EMG) data were collected from the contralateral first dorsal interosseous muscle via surface electrodes placed over these muscles in a belly-tendon montage. EMG activity was amplified using the Medelec Synergy EMG/EP system (Medelec, Oxford, UK), and the data were band-pass filtered at 10-2,000 kHz. The optimal scalp location, or the hot spot, was determined using a TMS system (Magstim Rapid
2 stimulator; Magstim Ltd., Carmarthenshire, UK) and a 70-mm figure-of-eight coil. The handle of the coil was oriented 45° posterior to the midline, so that the electromagnetic current would flow perpendicular to the central sulcus; the stimulator was then moved over the scalp in 1-cm increments [
6,
14]. Once the hot spot was identified, a single-pulse TMS was delivered to the location for determination of resting motor threshold (rMT), which was defined as the lowest intensity of stimulus necessary to produce MEPs ≥50 µV peak-to-peak amplitude in five out of ten consecutive trials. The muscle activity was carefully monitored by a real-time EMG, in order to confirm a relaxed state prior to stimulation [
15].
Repetitive transcranial magnetic stimulation
In each session, rTMS was applied to the M1 of the right target motor cortex area corresponding to the left hand, using a Magstim Rapid
2 stimulator with two booster modules. Real rTMS was delivered at 10 Hz and 90% rMT for 5 seconds with a 55-second inter-train interval. A total of 1,000 pulses were delivered over a period of 20 minutes. The stimulation was applied to the motor cortex by holding the figure-of-eight coil tangential to the skull. Sham rTMS was performed with the coil held at 90° from the scalp using the same stimulation parameters (duration, time, frequency) as with the real rTMS [
4]. The rTMS protocols used in the present study are in accordance with the safety guidelines for rTMS applications [
4].
Transcranial direct current stimulation
The tDCS was applied using a battery-driven DC stimulator (neuroConn GmbH, Ilmenau, Germany) over the right M1. A constant current flow of 1 mA was applied for 20 minutes through the wet sponge electrodes (size 7¡¿5 cm) positioned over the M1 and the contralateral supraorbital area. The M1 electrode was placed on the target site for cortical stimulation (the right M1). The supraorbital electrode was placed over the eyebrow, contralateral to the stimulated M1. The polarity of tDCS was established by the electrodes placed over the target M1. For example, the 'anodal tDCS', the anode was placed over the right M1, whereas the 'cathodal tDCS' involved positioning over the right M1. During the sham tDCS, the DC stimulator was activated at the beginning of the stimulation and then was gradually weakened after 5 seconds [
16].
Assessment of hand motor function
For evaluating the performance of hand motor function, each participant performed the Purdue Pegboard test [
17], the box and block test [
18], and the grip strength test [
18] with their left hand just before and immediately after each session.
Statistical analysis
For all statistical calculations, SPSS ver. 20.0 (IBM, Armonk, NY, USA) was used. A paired t-test was used to assess the statistical significance in the changes of MEP measurements and hand motor function tests, prior to and immediately after dual-mode stimulation in each condition. To assess differences in the time course of each stimulating condition, we used repeated measures ANOVA with post-hoc pairwise comparisons (Bonferroni corrected). The p-values less than 0.05 were considered statistically significant.
Go to :

RESULTS
No adverse effects were reported by the subjects during or after the stimulation. All subjects completed all four conditions.
Corticomotor excitability
The MEP amplitudes after stimulation were significantly higher than before stimulation in condition 1 (pre-stimulation, 871.99±345.48 mV; post-stimulation, 1,348.04±520.92 mV; p<0.001) and condition 2 (pre-stimulation, 935.49±307.77 mV; post-stimulation, 1,323.10±628.17 mV; p=0.002). There were no significant differences in MEP amplitudes before and after the stimulations in conditions 3 and 4. The MEP amplitude was higher in condition 2 than in both condition 3 (p=0.006) and condition 4 (p=0.001); while in condition 1, it was only higher than the condition 4 (p=0.007). The MEP latency values measured at pre- and post-stimulations were not significantly different in any of the conditions (
Fig. 2).
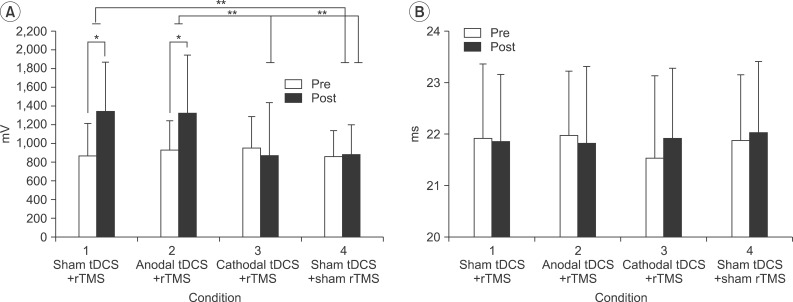 | Fig. 2(A) MEP amplitude and (B) MEP latency in the right M1 pre- and post-stimulations. Error bars represent the standard deviation for each condition. *p<0.05 between pre- and post-stimulation, **p<0.05 among conditions. rTMS, transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; M1, primary motor cortex; MEP, motor evoked potential. 
|
Hand motor function
The results of the Purdue Pegboard test showed significant improvement after the stimulations under condition 1 (pre-stimulation, 15.84±1.25; post-stimulation, 16.64±1.32; p=0.007) and condition 2 (pre-stimulation, 16.12±1.62; post-stimulation, 16.84±1.21; p=0.007). The results of the box and block test showed significant improvement after the stimulations under condition 1 (pre-stimulation, 62.88±7.60; post-stimulation, 66.20±7.92; p=0.001) and condition 2 (pre-stimulation, 64.60±7.14; post-stimulation, 67.56±9.4; p=0.01). For the grip strength test, there was no significant difference between pre- and post-stimulations in any of the conditions (
Fig. 3).
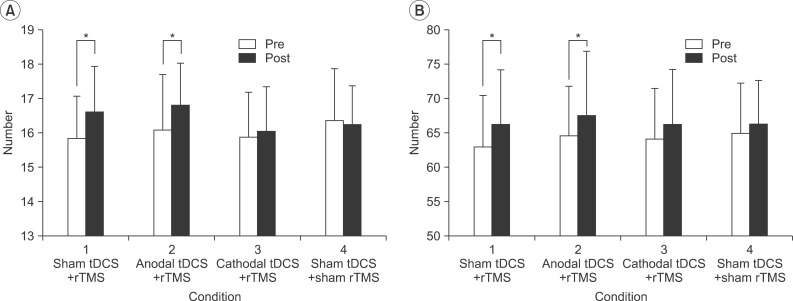 | Fig. 3(A) The Purdue Pegboard test and (B) the box and block test in the left hand, pre- and post-stimulations. Error bars represent the standard deviation for each condition. *p<0.05 between pre- and post-stimulations. rTMS, transcranial magnetic stimulation; tDCS, transcranial direct current stimulation. 
|
Go to :

DISCUSSION
This study was designed to investigate the interactive modulating effects of the high-frequency rTMS and simultaneously stimulating anodal or cathodal tDCS on the contralateral hemisphere. We concluded that the simultaneous stimulation of anodal tDCS over the left M1 and 10 Hz rTMS over the right M1 induced a neuromodulatory effect, which is interpreted by the interhemispheric interaction and homeostatic plasticity. This resulted in modulation of the rTMS effect on cortical excitability and hand motor function, as shown in condition 2 of this experiment.
By reducing cortical excitability in the dominant hemisphere, there may be an associated release of the contralateral motor cortex from suppression, which could explain the increase in cortical excitability in the non-dominant hemisphere [
19]. Recent studies have found that applying cathodal tDCS over the dominant M1 modulates an interhemispheric interaction resulting in an inhibitory effect [
8,
19,
20]. Siebner et al. [
21] investigated the effect of dual-mode stimulation on motor function, using sequential stimulation of tDCS and rTMS over the same M1. They found that facilitatory preconditioning of M1 by anodal tDCS augmented the inhibitory effect of low-frequency rTMS and decreased cortical excitability. On the other hand, the inhibitory preconditioning by cathodal tDCS counteracted the inhibitory effect of low-frequency rTMS and paradoxically increased cortical excitability [
21]. This paradoxical interaction of dual stimulation may be explained by homeostatic plasticity, which is a negative feedback-mediated form of plasticity that serves to maintain network activity at a desired set point [
12].
Our method of bihemispheric, dual-mode stimulation inducing interhemispheric interaction may modulate motor functions and corticomotor excitability through homeostatic plasticity. We expected that simultaneous cathodal or anodal tDCS over the dominant M1, with 10 Hz rTMS over the non-dominant M1, induced the interhemispheric modulation via transcallosal inhibitory fibers and resulted in facilitation or inhibition of rTMS effect on cortical excitability in the non-dominant M1. However, contrary to our expectation, results of the present study showed that the simultaneous cathodal tDCS over the contralateral M1 cancelled the facilitating effect of 10 Hz rTMS on cortical excitability and hand motor functions. Furthermore, the simultaneous anodal tDCS over the contralateral M1 did not reduce the effect of 10 Hz rTMS. These may be interpreted to be the result of homeostatic plasticity of the non-dominant M1, which interacted with the subsequent effect of rTMS on neuronal excitability of healthy individuals. To the best of our knowledge, our work is the first to report the interaction between transcallosal modulation and homeostatic plasticity on the modulation of rTMS effect.
Our study did not, however, demonstrate the augmentation of a facilitatory effect of unimodal high-frequency rTMS on motor function by dual-mode stimulation. There are two possible interpretations from these results. First, the contralateral anodal tDCS may decrease the cortical excitability of the target motor cortex through interhemispheric inhibition; therefore, the facilitatory effect of subsequent rTMS could not exceed the effect from rTMS only, due to a lower basal excitability state, even though the facilitation itself is higher than the condition with rTMS only. Second, it is possible that the modulatory effect on the target motor cortex from the contralateral tDCS was indirect and therefore weaker than the direct tDCS stimulation to the target motor cortex.
Further investigation on the different combinations of dual-mode, noninvasive brain stimulation could contribute to the discovery of better therapeutic tools for improving motor functions.
Go to :

ACKNOWLEDGMENTS
This study was supported by a National Research Foundation of Korea grant (No. 2011-0016960) funded by the Korean government, and by a KOSEF grant (No. M10644000022-06N4400-02210).
Go to :

Notes
Go to :

References
1. Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Curra A, Gilio F, et al. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998; 122:79–84. PMID:
9772114.

2. Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998; 15:333–343. PMID:
9736467.

3. Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000; 111:800–805. PMID:
10802449.

4. Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006; 37:1471–1476. PMID:
16675743.

5. Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008; 1:206–223. PMID:
20633386.

6. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001; 57:1899–1901. PMID:
11723286.

7. Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003; 114:600–604. PMID:
12686268.

8. Vines BW, Nair D, Schlaug G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur J Neurosci. 2008; 28:1667–1673. PMID:
18973584.

9. Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006; 17:671–674. PMID:
16603933.

10. Netz J, Ziemann U, Homberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995; 104:527–533. PMID:
7589304.

11. Gorsler A, Baumer T, Weiller C, Munchau A, Liepert J. Interhemispheric effects of high and low frequency rTMS in healthy humans. Clin Neurophysiol. 2003; 114:1800–1807. PMID:
14499741.

12. Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009; 10:861–872. PMID:
19888284.

13. Mordillo-Mateos L, Turpin-Fenoll L, Millan-Pascual J, Nunez-Perez N, Panyavin I, Gomez-Arguelles JM, et al. Effects of simultaneous bilateral tDCS of the human motor cortex. Brain Stimul. 2012; 5:214–222. PMID:
21782545.

14. Nitsche MA, Roth A, Kuo MF, Fischer AK, Liebetanz D, Lang N, et al. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci. 2007; 27:3807–3812. PMID:
17409245.

15. Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994; 91:79–92. PMID:
7519144.

16. Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998; 9:2257–2260. PMID:
9694210.

17. Wade DT. Measuring arm impairment and disability after stroke. Int Disabil Stud. 1989; 11:89–92. PMID:
2698395.

18. Boissy P, Bourbonnais D, Carlotti MM, Gravel D, Arsenault BA. Maximal grip force in chronic stroke subjects and its relationship to global upper extremity function. Clin Rehabil. 1999; 13:354–362. PMID:
10460123.

19. Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008; 9:103. PMID:
18957075.

20. Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992; 453:525–546. PMID:
1464843.

21. Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004; 24:3379–3385. PMID:
15056717.

Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download