CASE REPORT
A 52-year-old male with a history of hypertension for 3 years and a smoking history of 30 pack/year suffered an electrical shock on his right hand from a contact with a 220 V of alternating current. It was presumed that the current entered his right hand and exited through his left hand. He did not have any significant symptoms immediately after the shock, so he did not seek treatment. After 1 week, however, he perceived weakness in both upper extremities. The strength of his left arm gradually improved, but the weakness in his right arm progressed. The patient visited our hospital 2 weeks post-injury.
According to the manual muscle test (MMT; Medical Research Council), both lower extremities were normal, but an examination of the right upper extremity revealed shoulder flexion and abduction (2/5), elbow flexion and extension (3/5), wrist flexion and extension (3/5), and finger extension (2/5). Motor power in his left upper extremity was graded as 5/5, except for the shoulder flexion and abduction (4/5) and wrist extension (4/5). Nerve conduction study (NCS) and electromyography were performed 4 weeks post-injury, and motor power of the right side improved to 4/5, except for the right index finger extensor (3/5). Sensory function was intact, muscle stretch reflexes were normoactive, no pathological reflexes were identified, and all the other cranial nerve and cerebellar functions were normal. A sensory NCS was normal but a motor NCS revealed delayed-onset latency and reduced amplitude of compound muscle action potentials in the right radial nerve (
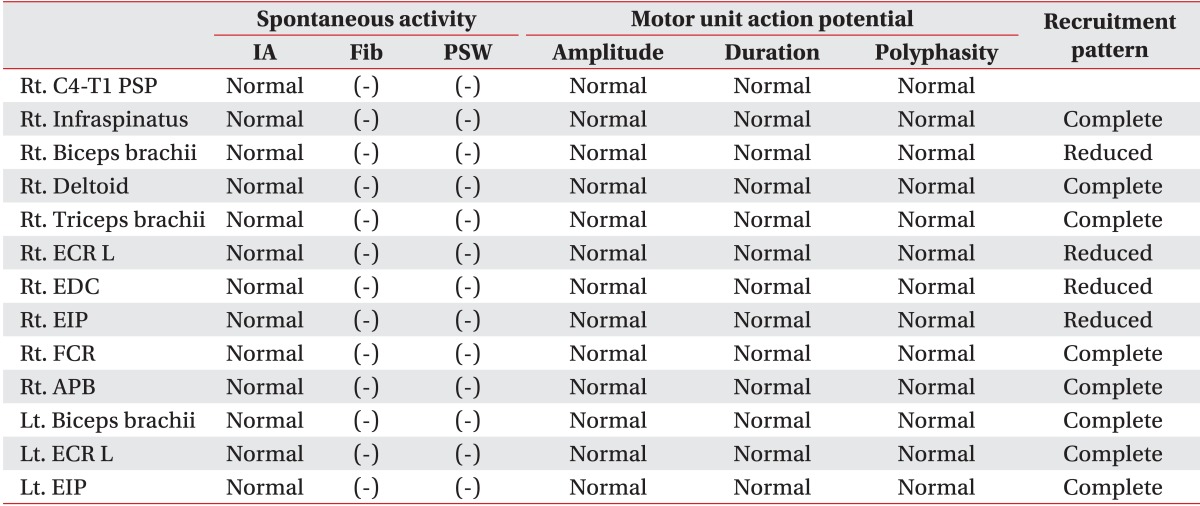
Table 1). There were no abnormal spontaneous activities during the needle electromyography, but a reduced recruitment of motor unit action potentials was observed in the right elbow flexor, wrist extensor, and finger extensor muscles (
Table 2). The patient was diagnosed with an incomplete right radial nerve injury and received physical therapy at another hospital.
Table 1
Results of nerve conduction studies
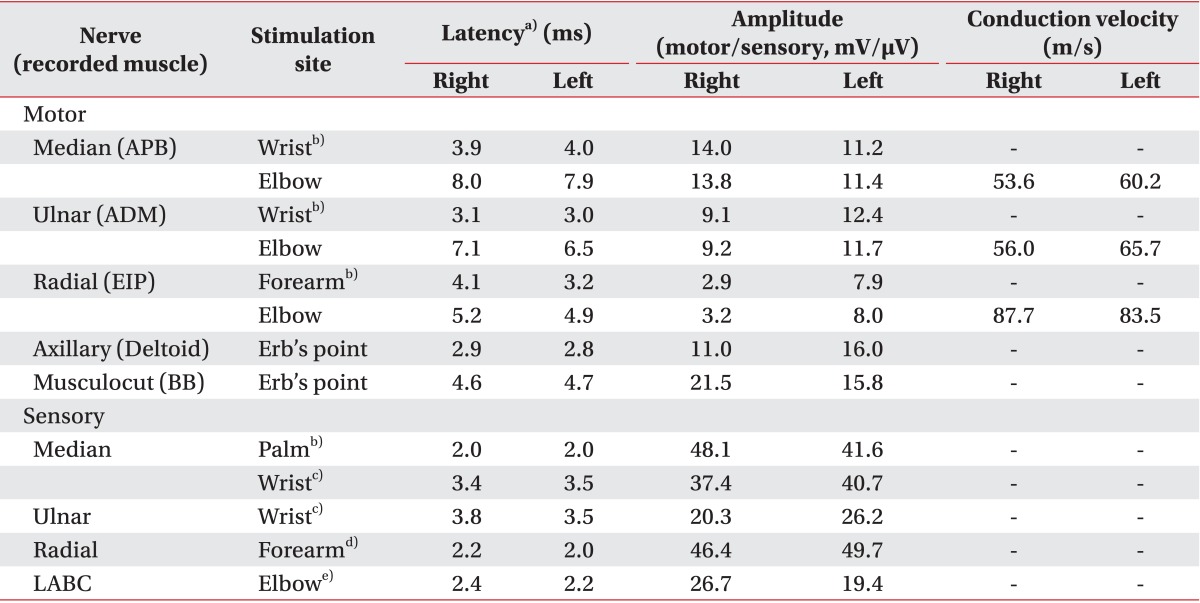

Table 2
Needle electromyography of muscles in the upper extremities

At 5 weeks after the injury, the patient visited an emergency department for a sudden-onset of weakness on the right-side with an aphasia. He had central-type facial palsy and weakness of the right upper and lower extremities, with proximal MMT grade of 3/5 and distal MMT grade of 2/5. His muscle stretch reflexes were hyperactive in the right upper and lower extremities, and Hoffman sign was observed on his right side. Laboratory results were within the normal ranges, except for the increased liver enzymes (aspartate aminotransferase/alanine aminotransferase [AST/ALT], 32/71 IU/L) and dyslipidemia (total cholesterol, 263 mg/dL; high-density lipoprotein-cholesterol, 38 mg/dL; low-density lipoprotein-cholesterol, 158 mg/dL; triglyceride, 217 mg/dL). Holter monitoring for 24 hours revealed a normal sinus rhythm, an echocardiogram revealed mild-to-moderate left ventricular hypertrophy, and a brain magnetic resonance imaging scan revealed an acute cerebral infarction of the left middle cerebral artery (MCA) (
Fig. 1A, B). Additionally, magnetic resonance angiography showed marked focal stenosis in the left MCA and proximal anterior cerebral artery, as well as stenosis of the proximal basilar artery and right proximal cervical internal carotid artery (
Fig. 1C, D). Because the patient visited the hospital 18 hours after the attack, oral aspirin was administered. He received rehabilitation treatment including physical, occupational, and speech therapies. One week after the admission, his right-side motor power had improved in the upper extremity (3/5), proximal lower extremity (4/5), and distal lower extremity (3/5). He was discharged 2 weeks after the admission (Korean Modified Barthel Index score, 91).
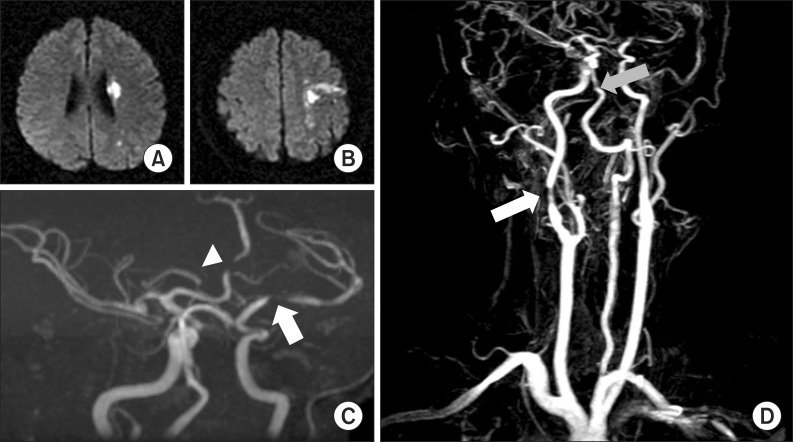 | Fig. 1(A, B) The T2-weighted brain magnetic resonance imaging scan revealed ischemia in left middle cerebral artery territory. (C) Magnetic resonance angiography (MRA) identified marked focal stenosis in the left M1 mid-portion (white arrow) and mid-A2 (white arrowhead). (D) MRA also revealed stenosis of the proximal basilar artery (gray arrow) and the right proximal cervical internal carotid artery (white arrow). 
|
Go to :

DISCUSSION
Various neurological symptoms, such as neuropathy or reflex sympathetic dystrophy, can occur in the subacute period following an electric injury [
2]. Among the PNS injury, the median nerve is the most frequently injured nerve followed by the ulnar nerve. In one case, a patient had an injured common peroneal nerve [
3]. The patient described herein suffered a radial nerve injury, not reported in this study, possibly due to the entrance and exit sites. However, among the 28 high-voltage electric injury cases reported, eight had peripheral nerve injury that most frequently involved the radial and ulnar nerves in the upper extremities [
4].
The severity of electric injury is determined by voltage, amperage, type of current (alternating or direct), resistance of the body, path of electrical flow, and duration of contact; type of current and voltage are the most important factors [
4]. The resistance of body tissue decreases according to bone, fat, ligaments, skin, muscles, vessels, and nerves. High-voltage current passes through the body across the shortest distance regardless of tissue type, and it may cause massive soft tissue injury and extensive skin necrosis at the contact site [
4,
5]. However, low-voltage current tends to transmit through tissues with low resistance, such as vessels and CNS tissue. This explains why low currents can induce fatal injuries, such as ventricular fibrillation and cardiac arrest without skin lesions. Additionally, alternating current is more hazardous than the direct current, because tetanic muscular contractions fix the subject to the source of electricity [
6]. Therefore, direct currents below 220 V are rarely fatal, whereas alternating current can be lethal, particularly in cases where the voltage is less than 50 V.
Neurological complications may develop immediately or they can develop later on after the electric injury. A retrospective study of the neurological consequences of electrical burns revealed that 17% of those who sustained a high-voltage electric injury experienced delayed neuropathies [
7]. The latency period varies from several days to even decades, and different mechanisms of injury may contribute to this type of delayed damage, including thermal damage, sympathetic stimulation, vascular damage, histological or electrophysiological changes, and direct mechanical trauma. Thermal injury to perineural tissue results in progressive perineural fibrosis, which in turn results in delayed-onset neuropathies.
Cerebral infarction is rarely reported, although the infarction of watershed areas vulnerable to ischemia may result from cardiopulmonary arrest. Cerebral infarctions without hemodynamic alterations have been reported following electric injury [
8,
9], but the underlying mechanisms are poorly understood [
8,
9,
10]. Two cases of cerebral infarction from a direct electrical injury exhibited wedge-shaped infarctions of the fronto-temporal and parietal lobes, respectively. These were probably caused by territory occlusion due to vasospasm or embolism formation [
8,
9]. The current case differs in that the ischemic stroke occurred in the left MCA territory, and the patient already had a stenosis of that artery. It is possible that vascular endothelial damage and thrombus formation obstructed the narrowed vessel [
10].
The current patient experienced weakness in his upper extremities at 1 week post-injury, which later developed into incomplete right radial neuropathy and a cerebral infarction, 5 weeks after the accident. We could not determine whether the electric injury was the primary etiology of the stroke, because he had other risk factors including hypertension, smoking, and dyslipidemia. However, low-voltage electric injury can affect an individual with vulnerable cerebral vasculature, and can result in cerebral arterial constriction, endothelial damage, and thrombus formation.
If a patient suffering from electric injury complains of weakness, clinicians should consider various causes and carefully perform the neurological examination, and should utilize electrophysiological or imaging techniques to rule out peripheral neuropathy, myelopathy, and cerebral damage. It is important that physicians recognize that delayed neurological sequelae may develop following the electric injury, and the patients should be followed up until they achieve medical and neurological stability.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download