Abstract
Systemic lupus erythematosus (SLE) is an autoimmune connective tissue disease characterized by multiorgan involvement with diverse clinical presentations. Central nervous system involvement in neuropsychiatric syndromes of SLE (NPSLE), such as cerebrovascular disease and myelopathy, is a major cause of morbidity and mortality in SLE patients. The concomitant occurrence of myelopathy, cerebrovascular disease, and peripheral neuropathy in a patient with SLE has not yet been reported. We report on a 41-year-old woman with SLE who showed motor and sensory impairment with urinary retention and was diagnosed with cervical myelopathy and acute cerebral infarction by spine and brain magnetic resonance imaging and peripheral neuropathy by electrodiagnostic examination. Even though pathogenesis of NPSLE is not well elucidated, we assume that increased antibodies of anti-double stranded DNA (anti-dsDNA), presence of lupus anticoagulant and hypertension are risk factors that have caused neuropsychiatric lupus in this patient.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of autoantibodies to components of the cell nucleus, and has diverse clinical manifestations. The prevalence of lupus in Koreans has been found to be similar to that in Caucasians. Women comprise approximately 90% of lupus patients, among half of them showing first symptom at 15 to 20 years old, and most of them showing the disease from 15 to 44 years old [1]. Neuropsychiatric systemic lupus erythematosus (NPSLE) has been developed for all 19 syndromes, including the neurologic syndromes of the central, peripheral and autonomic nervous system and psychiatric syndromes observed in patients with SLE, in which other causes have been excluded. NPSLE may precede lupus or may occur during any phases, including the active and latent phase. Its neurological symptoms vary depending on the patient. Some show a single symptom, while some show multiple symptoms that occur in various organs [2].
According to a previous report, the prevalence of NPSLE in SLE patients is varied. Among SLE patients, the central system involvement is about 40% [3]. Stroke occurred in 3%-30% of the patients, presenting from focal abnormality to generalized cerebral dysfunction [4]. Myelopathy is one of the less common neuropsychiatric manifestations of SLE (1%-3% of patients), with substantial neurologic symptoms, such as paraplegia or tetraplegia [5]. Peripheral nervous system involvement in SLE patients accounts for 13.5%. Of these SLE-related cases comprise approximately 60.4% with symptoms, such as muscular weakness, sensory impairment, pain and hyporeflexia [6].
To our knowledge, this is the first case report on a concomitant occurrence of cervical myelopathy, cerebral infarction, and peripheral neuropathy in an SLE patient, along with literature review.
A 41-year-old woman visited the emergency room at our hospital, complaining of muscle weakness in the limbs, hypoesthesia in the lower extremity, and abdominal distention caused by acute urinary retention. At the age of 26, the patient had a renal biopsy due to generalized edema, multiple arthralgia and anemia, and was diagnosed with type-IV lupus nephritis. Since then she had been followed up with medication for fifteen years. The patient was catheterized for acute urinary retention soon after visiting the emergency room, where she passed 2,300 mL of urine. She was then hospitalized in the Department of Neurology for assessment and treatments. High signal intensity was observed around the 5th and 6th cervical spinal cord (Fig. 1A) on T2-weighted spinal magnetic resonance imaging (MRI), performed on the first day of hospitalization; and one month later, they were extended from the 3rd to 6th cervical spinal cord (Fig. 1B), which manifested cervical myelopathy. In addition high signal intensity on diffusion weighted brain MRI (Fig. 2A) and low signal intensity on the apparent diffusion coefficient map (Fig. 2B) were observed in the left internal capsule, which manifested acute cerebral infarction. On fluid attenuated inversion recovery imaging, high signal intensity was observed around the medulla oblongata and bilateral cerebellum (Fig. 2C), which manifested multiple cerebral vasculitis. Abnormalities were not observed on the cerebrospinal fluid examination, and also the oligoclonal band was not observed. The immunological test showed an increase in double-stranded DNA (dsDNA) antibodies (152 IU/mL; reference value, 0-7 IU/mL) and an increase in lupus anticoagulant (one of antiphospholipid antibodies; 71.8 seconds; reference value, 31-44 seconds); and the anticardiolipin antibody test was negative. The manual muscle test (MMT) was conducted on the basis of the Medical Research Council (MRC) grading, whereof results were as follows. Voluntary muscle contraction was not observed in the right upper and lower extremities. In the left upper extremity, muscle strength was graded 2/5 at the level from the 5th cervical spinal cord to the 1st thoracic spinal cord. In the left lower extremity, it was graded 1/5 at the level from the 2nd lumbar spinal cord to the 1st sacral spinal cord. With respect to hypoesthesia, electromyography was performed in its early stage. On the sensory nerve conduction study, it showed low amplitudes in the right median and ulnar nerves stimulation and no response in the bilateral sural nerves stimulation (Table 1). On the motor nerve conduction study, it showed low amplitudes in the right median and ulnar nerves stimulation and no response in the bilateral tibial and peroneal nerves stimulation. With respect to the right median and ulnar nerves, F-waves were within the normal range, which manifested peripheral neuropathy (Table 2). Such findings show a case of NPSLE defined by the American College of Rheumatology; specifically, a concurrence of myelopathy, cerebrovascular disease and peripheral neuropathy [2]. After being treated with steroid pulse therapy, prednisolone, immunosuppressants, and plasma exchange for five months, the patient was transferred to the Department of Neurology at the hospital. Following two months of medication, seven months after the occurrence of symptoms, she was retransferred to the Department of Rehabilitation Medicine. On the physical examination, conducted on the first day of transfer, was shown mild dysarthria, declines in gag and cough reflex and left-sided tongue deviation. In the right upper and lower extremities, deep tendon reflex was hyperreflexive, but spasticity was not observed. Both sides tested positive on the Babinski and Hoffman test, but ankle clonus was not observed. Light touch and pinprick sensation was impaired below the level of the 4th cervical spinal cord, on both sides. Also hypoesthesia occurred around the anus. Voluntary anal contraction was possible, but anal sphincter showed hypotonous. The bulbocavernosus reflex was absent. MMT was conducted again seven months after the onset of disease. In the right part, muscular strengths were graded: 4/5 from the 5th cervical spinal cord to the 7th one; 3/5 from the 8th cervical spinal cord to the 1st thoracic spinal cord; 1/5 from the 2nd lumbar spinal cord to the 5th one; and 2/5 at the 1st sacral spinal cord, respectively. In the left part, they were graded: 3/5 at the level from the 5th cervical spinal cord to the 1st thoracic spinal cord; 2/5 from the 2nd lumbar spinal cord to the 3rd one; 1/5 from the 4th lumbar spinal cord to the 5th one; and 2/5 at the 1st sacral spinal cord, respectively. Altogether muscle weakness was manifested in the four extremities. The patient could roll side to side by herself, but had a poor sense of dynamic sitting balance. Also the patient was able to urinate by herself with residual urine of 100 to 200 mL, but had poor control of sphincter. Independent defecation was observed. The Korean-version Modified Barthel Index, evaluated seven months and nine months after the onset of disease, increased from 15 to 27, out of 100. The spinal cord independence measure increased from 15 to 32, out of 100. At nine months after the onset of disease, neurologic changes were not observed except for the spasticity being graded 1 on the four extremities (the Modified Ashworth Scale) and bulbocavernosus reflex being present.
A definite evidence for the pathophysiology of NPSLE has not been established, but ordinarily it appears that NPSLE occurs with the interaction among vascular or perivascular injury caused by immune complexes, vascular or neuronal injury caused by autoantibodies and vascular or neuronal injury caused by locally produced cytokine and ensuing cellular immune-mediated responses [7]. Vasculitis and spinal ischemic necrosis caused by arterial thrombosis are known to be the pathogenesis of myelopathy [5]. It is also proposed that an increase in anti-dsDNA antibodies and the existence of lupus anticoagulant are related to the invasion of the central nervous system in lupus patients. Such antibodies were found to be associated with the hypercoagulable state, vasculopathy and thrombokinesis [3]. In addition it has been reported that variable risk factors, such as hypertension, carotid vasculopathy, and dyslipidemia, are associated with cerebrovascular accidents and the decline in cognitive function [8]. The current patient had a risk factor of hypertension. She seemed to develop various neuropsychiatric lupus syndromes at the same time due to vasculitis, the increase in anti-dsDNA antibodies and the hypercoagulable state created by lupus anticoagulant.
Myelopathy is one of the less common neuropsychiatric manifestations of SLE (1%-3% of patients) [5]. Myelopathy should be suspected with the patients showing bilateral weakness of legs with or without arms (paraplegia/tetraplegia), neurogenic bowel and bladder dysfunction and sensory impairment with cord level similar to that of motor weakness and when one of them progresses rapidly. In the majority of cases, myelitis occurs shortly after the diagnosis of SLE, usually within the first five years from the onset of the disease. At least one recurrence of myelitis in SLE is noted in 21%-55% of patients. Especially episodes of recurrence were found mainly in untreated patients and in patients receiving long-term therapy with low or medium doses of glucocorticosteroids [5]. This patient had taken low-dose prednisolone for a long period following the diagnosis of lupus, and the disease activity had been stable. Fifteen years later, however, myelitis occurred. This is a comparatively rare case that deserves to be reported. When the symptoms first developed, myelopathy was observed at the level from the 5th to the 6th cervical spinal cord. One month later, however, it extended even to the level from the 3rd to the 6th cervical spinal cord, which might be supposedly caused by the fact that the dosage of prednisolone was reduced due to generalized edema, a side-effect from steroid pulse therapy.
It is reported that stroke occurs in 3%-30% of patients with SLE, most of which are ischemic strokes. Cerebral hemorrhage in SLE has been reported in 0.4%-7% of patients with SLE [4,9]. The pathophysiology of stroke in patients with SLE can be explained with cardiogenic thromboembolism, antibody-mediated hypercoagulable state, and cerebral vasculitis, against which anticoagulant therapy is widely used [4]. In the present case, the antibody-mediated hypercoagulable state, created by anti-dsDNA antibodies and lupus anticoagulant, is presumed to be a possible cause of acute cerebral infarction. Cerebral vasculitis, detected on brain MRI, may present another cause.
Finally neuropsychiatric SLE, which involves the peripheral nervous system, shows clinical symptoms, such as distal or proximal weakness and/or sensory loss of extremities, pain, hyporeflexia and sensation changes, most of which are known to be relevant to the disease activity index. Florica et al. [6], who conducted a study on a total of 1,533 patients with SLE, reported that a total of 207 patients (13.5%) experienced at least one peripheral nervous system manifestation. Of these 125 patients (60.4%) were found to be SLE-related. The most common peripheral neuropathy observed was peripheral polyneuropathy, with a predominance of the sensory form (36.7%), followed by sensory motor variant (18.8%), a cranial neuropathy (12.5%), and peripheral mononeuropathy (11.1%). The main findings on nerve conduction studies were signs of axonal neuropathy in the majority (78%) of the patients. In particular it tended to involve the lower extremities rather than the upper and also to involve only one side [6]. In the present case, latency and conduction velocity were judged well-preserved on the sensory and motor nerve conduction studies. However they were referred to as sensory motor variant neuropathy and axonal neuropathy, characterized by low amplitudes. Considering the side effects from antimalarial drugs (hydroxychloroquine) and immunosuppressants (azathioprine) as well as glucose intolerance and steroid-induced diabetes resulting from the prolonged administration of steroid, this patient was more likely to develop peripheral polyneuropathy [6]. In our case, however, the most notable characteristic was that the glucose level was within the normal range, that symptoms occurred acutely the same as NPSLE and that there was no temporal causality between the occurrence of symptoms and the administration of hydrochloroquine and azathioprine. In this context, the focus was shifted to the possibility of SLE-related peripheral polyneuropathy.
There is currently no report on the concurrence of myelitis, cerebral infarction, and peripheral polyneuropathy in patients with NPSLE. A previous study reported a case where the brain lesion gradually progressed subsequent to the onset of myelitis. At first a brain lesion was accidentally detected on brain single-photon emission computed tomography (SPECT) but was not observed on brain MRI, and there was no symptom that manifested cerebral disorders [8]. This patient showed neurological abnormalities and symptoms, such as motor weakness, hypoesthesia and dysuresia, which were proved by brain and spinal MRI scans and electromyogram. It confirmed the concurrence of cervical myelopathy, acute cerebral infarction and peripheral polyneuropathy, which has not been reported yet.
The prognoses of patients who have contracted SLE concomitant with myelopathy vary from full recovery to death. It may be helpful to inject intravenous cyclophosphamide and high-dose glucocorticosteroid at first, and later it may be effective to administer oral glucocorticosteroid for an extended period. In case muscular strength is graded 3/5 or higher on MRC grading on the first day of hospitalization or medical intervention is aggressively conducted within two weeks after the occurrence of symptoms, the patient can be expected to have a good prognosis [5]. This patient was treated with steroid pulse therapy within two weeks after the occurrence of symptoms, but she could not be kept under high-dose glucocorticosteroid due to generalized edema, a side effect from the oral administration of prednisolone. The patient was predicted to have an unfavorable prognosis, given the fact that the extent of myelopathy and stroke had spread further on follow-up MRI, and that she had not been rehabilitated for seven months after the occurrence of symptoms.
References
2. American College of Rheumatology. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999; 42:599–608. PMID: 10211873.
3. van Dam AP. Diagnosis and pathogenesis of CNS lupus. Rheumatol Int. 1991; 11:1–11. PMID: 1866569.

4. Futrell N, Millikan C. Frequency, etiology, and prevention of stroke in patients with systemic lupus erythematosus. Stroke. 1989; 20:583–591. PMID: 2718197.

5. Łukjanowicz M, Brzosko M. Myelitis in the course of systemic lupus erythematosus: review. Pol Arch Med Wewn. 2009; 119:67–72. PMID: 19341181.

6. Florica B, Aghdassi E, Su J, Gladman DD, Urowitz MB, Fortin PR. Peripheral neuropathy in patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2011; 41:203–211. PMID: 21641018.

7. Stahl HD, Ettlin TH, Plohmann A, Radu EW, Muller-Brand J, Steiger U, et al. Central nervous system lupus: concomitant occurrence of myelopathy and cognitive dysfunction. Clin Rheumatol. 1994; 13:273–279. PMID: 8088073.
8. Govoni M, Bombardieri S, Bortoluzzi A, Caniatti L, Casu C, Conti F, et al. Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a risk profile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology (Oxford). 2012; 51:157–168. PMID: 22075066.

9. Liveson JA, Ma DM. Laboratory reference for clinical neurophysiology. Philadelphia, PA: F.A. Davis;1992.
Fig. 1
A cervical spine magnetic resonance imaging of the patient. Sagittal T2-weighted image shows a high signal intensity lesion (arrow) in C5-6 spinal cord at onset (A) and C3-6 one month later (B).
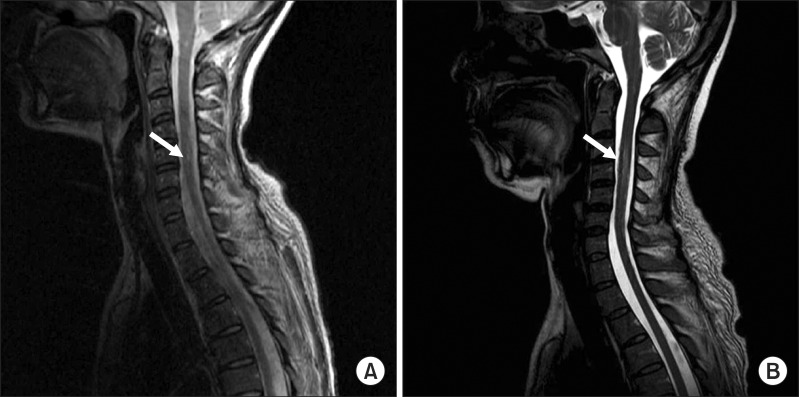
Fig. 2
A brain magnetic resonance imaging of the patient. Axial diffusion-weighted image shows high signal intensity (short arrow) in left internal capsule (A), with low signal intensity (arrowhead) in apparent diffusion coefficient map (B), showing acute cerebral infarction in this lesion. In flair image, there are high signal intensities (long arrow) in pons and both cerebella, suggesting multiple cerebral vasculitis (C).
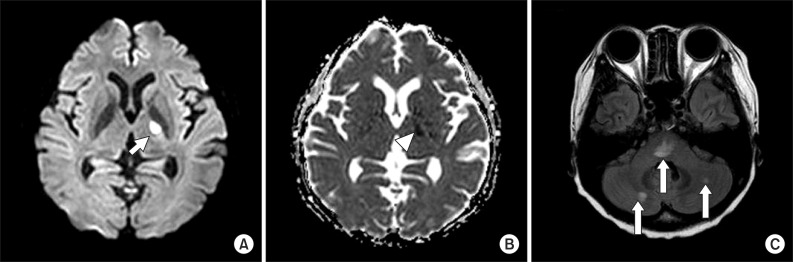
Table 2
Motor nerve conduction and F-wave study
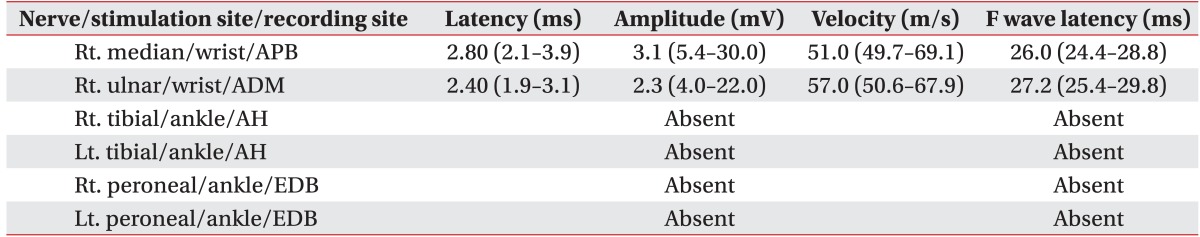
Reference values [9] are presented in brackets.
APB, abductor pollicis brevis; ADM, abductor digiti minimi; AH, abductor hallucis; EDB, extensor digitorum brevis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download