1. Sakini RA, Abdul-Zehra IK, Al-Nimer MS. Neuropathic manifestations in rheumatoid arthritis: a clinical and electrophysiological assessment in a small sample of Iraqi patients. Ann Saudi Med. 2005; 25:247–249. PMID:
16119528.

2. Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002; 29:62–67. PMID:
11824973.
3. Johnson RL, Smyth CJ, Holt GW, Lubchenco A, Valentine E. Steroid therapy and vascular lesions in rheumatoid arthritis. Arthritis Rheum. 1959; 2:224–229. PMID:
13662216.

4. Good AE, Christopher RP, Koepke GH, Bender LF, Tarter ME. Peripheral neuropathy associated with rheumatoid arthritis: a clinical and electrodiagnostic study of 70 consecutive rheumatoid arthritis patients. Ann Intern Med. 1965; 63:87–99. PMID:
14305971.
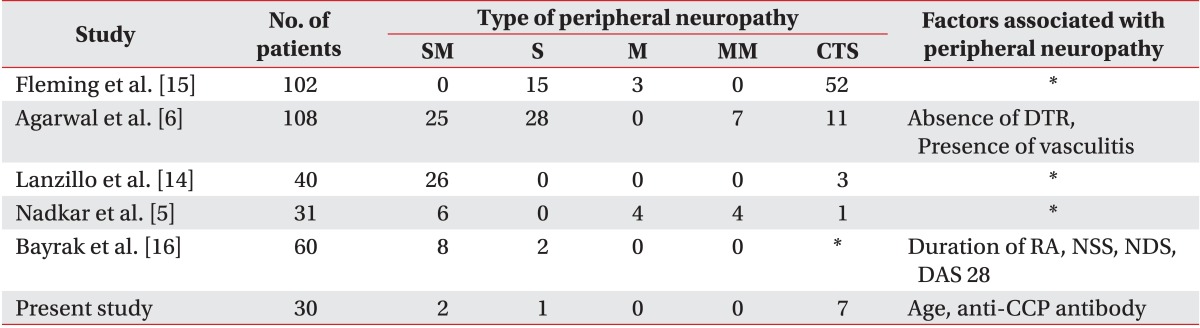
5. Nadkar MY, Agarwal R, Samant RS, Chhugani SJ, Idgunji SS, Iyer S, et al. Neuropathy in rheumatoid arthritis. J Assoc Physicians India. 2001; 49:217–220. PMID:
11225133.
6. Agarwal V, Singh R, Wiclaf , Chauhan S, Tahlan A, Ahuja CK, et al. A clinical, electrophysiological, and pathological study of neuropathy in rheumatoid arthritis. Clin Rheumatol. 2008; 27:841–844. PMID:
18084807.

7. Golding DN. Rheumatoid neuropathy. Br Med J. 1971; 2:169. PMID:
4103337.

8. Pouget J. Vascular neuropathies. Rev Prat. 2000; 50:749–752. PMID:
10853555.
9. Salih AM, Nixon NB, Gagan RM, Heath P, Hawkins CP, Dawes PT, et al. Anti-ganglioside antibodies in patients with rheumatoid arthritis complicated by peripheral neuropathy. Br J Rheumatol. 1996; 35:725–731. PMID:
8761183.

10. El M, Ashour S, Moustafa H, Ahmed I. Altered levels of soluble adhesion molecules in patients with rheumatoid arthritis complicated by peripheral neuropathy. J Rheumatol. 2002; 29:57–61. PMID:
11824972.
11. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–324. PMID:
3358796.

12. Dyck PJ, Sherman WR, Hallcher LM, Service FJ, O'Brien PC, Grina LA, et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980; 8:590–596. PMID:
7212646.
13. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62:2569–2581. PMID:
20872595.

14. Lanzillo B, Pappone N, Crisci C, di Girolamo C, Massini R, Caruso G. Subclinical peripheral nerve involvement in patients with rheumatoid arthritis. Arthritis Rheum. 1998; 41:1196–1202. PMID:
9663475.

15. Fleming A, Dodman S, Crown JM, Corbett M. Extra-articular features in early rheumatoid disease. Br Med J. 1976; 1:1241–1243. PMID:
1083759.

16. Bayrak AO, Durmus D, Durmaz Y, Demir I, Canturk F, Onar MK. Electrophysiological assessment of polyneuropathic involvement in rheumatoid arthritis: relationships among demographic, clinical and laboratory findings. Neurol Res. 2010; 32:711–714. PMID:
20307377.

17. Beghi E, Monticelli ML. Italian General Practitioner Study Group (IGPST). Chronic symmetric symptomatic polyneuropathy in the elderly: a field screening investigation of risk factors for polyneuropathy in two Italian communities. J Clin Epidemiol. 1998; 51:697–702. PMID:
9743318.
18. Weller RO, Bruckner FE, Chamberlain MA. Rheumatoid neuropathy: a histological and electrophysiological study. J Neurol Neurosurg Psychiatry. 1970; 33:592–604. PMID:
4320255.

19. Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004; 17:309–318. PMID:
15355943.

20. Kroot EJ, de Jong BA, van Leeuwen MA, Swinkels H, van den Hoogen FH, van't Hof M, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000; 43:1831–1835. PMID:
10943873.

21. van Gaalen FA, van Aken J, Huizinga TW, Schreuder GM, Breedveld FC, Zanelli E, et al. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum. 2004; 50:2113–2121. PMID:
15248208.

22. Ceccato F, Roverano S, Barrionuevo A, Rillo O, Paira S. The role of anticyclic citrullinated peptide antibodies in the differential diagnosis of elderly-onset rheumatoid arthritis and polymyalgia rheumatica. Clin Rheumatol. 2006; 25:854–857. PMID:
16514472.

23. Turesson C, Jacobsson LT, Sturfelt G, Matteson EL, Mathsson L, Ronnelid J. Rheumatoid factor and antibodies to cyclic citrullinated peptides are associated with severe extra-articular manifestations in rheumatoid arthritis. Ann Rheum Dis. 2007; 66:59–64. PMID:
16901955.

24. Kim SK, Park SH, Shin IH, Choe JY. Anti-cyclic citrullinated peptide antibody, smoking, alcohol consumption, and disease duration as risk factors for extraarticular manifestations in Korean patients with rheumatoid arthritis. J Rheumatol. 2008; 35:995–1001. PMID:
18464311.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download