Abstract
Objective
To investigate the effect of deep brain stimulation (DBS) on reducing dystonia and disability in adults with cerebral palsy (CP) and to compare the therapeutic outcomes between primary dystonia patients and CP patients over two years after bilateral pallidal DBS.
Methods
Five patients with primary dystonia and seven CP patients with dystonia were recruited. All subjects received DBS surgery in both globus pallidus. Burke-Fahn-Marsden dystonia rating scale consisting of dystonia movement score and disability score and subjective satisfaction scale were assessed after 1 month and every 6 months over two years following DBS treatment.
Results
On the dystonia movement scale, both groups of primary dystonia patients and CP patients showed a significant decrease over time following DBS. On the disability scale, patients with primary dystonia showed a significant decrease over time, whereas the disability score of CP patients did not change over the two years. Comparing the dystonia movement and disability scores of CP patients at each assessment, patients with primary dystonia showed a significant reduction after 6 months. Comparing the satisfaction scores of CP patients after DBS, patients with primary dystonia showed significantly higher subjective satisfaction.
Dystonia is a neurological movement disorder which shows involuntary muscle contractions. Sustained muscle contractions trigger repetitive abnormal movements or abnormal postures [1,2]. Dystonia may occur idiopathically or as a secondary symptom due to a certain type of disease or condition [3]. It can be treated with dopaminergic drugs, anti-cholinergic drugs, or botulinum toxin muscle relaxants [1,4]. Neuroablative surgery, such as thalamotomy or pallidotomy and deep brain stimulation (DBS), can be performed if generalized dystonia does not respond to conservative treatment [5,6].
Over the past decade, DBS has emerged as a major therapeutic modality for movement disorders, such as Parkinson disease (PD), essential tremor and dystonia [7,8,9,10]. Particularly, DBS in the globus pallidus interna (GPi) has been consistently reported to be significantly beneficial in patients with primary generalized dystonia [11,12]. As such, DBS has raised hope for the treatment of cerebral palsy (CP) patients with dystonia who suffer from similar symptoms as patients with primary dystonia [13].
However, patients with secondary dystonia have been known to respond less well to DBS than patients with primary dystonia [14,15,16]. Dystonia caused by CP or other brain injury responded much less effectively among patients with secondary dystonia, even though the one-year outcome in a prospective pilot study showed that bilateral pallidal DBS treatment has beneficial effects for CP patients with dystonia-choreoathetosis [13]. Moreover, few results have been reported regarding the long-term effects of DBS in CP patients with dystonia.
The purpose of this study was to investigate whether the long-term effects of DBS in GPi on reducing dystonia and disability are present in adults with CP. Then, therapeutic outcomes are compared between primary dystonia patients and CP patients over two years following bilateral pallidal DBS treatment.
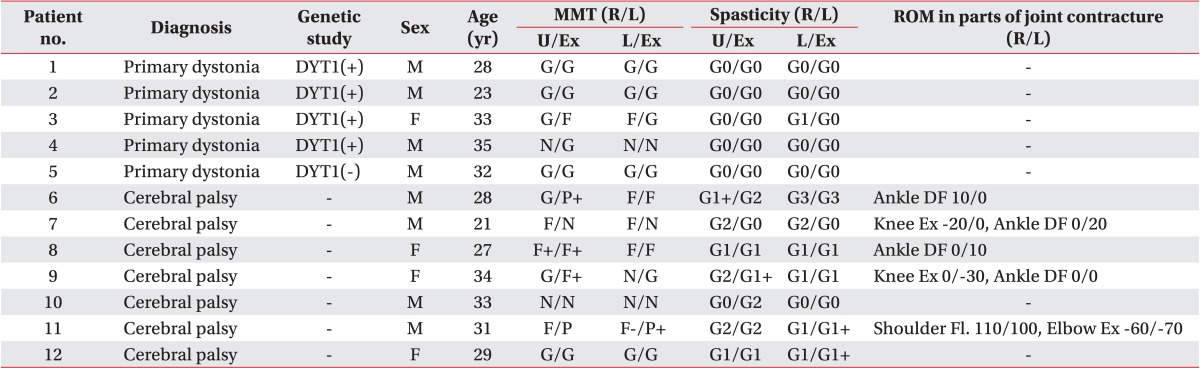
Five patients with primary dystonia and seven CP patients with dystonia were recruited in our study (Table 1). Of five patients with primary dystonia, four patients had DYT1 positive dystonia and one patient had DYT1 negative dystonia. The seven adults with CP had a neonatal history of hypoxic or ischemic brain injury and a developmental history of delayed motor milestones, such as head control, sitting, and walking. All subjects had disabling dystonia, defined as involuntary and sustained muscle contractions which lead to abnormal movements or abnormal postures. The dystonia was generalized with a combination of trunk dystonia and involvement of any other segments including face, neck, or limbs. The characteristics of the subjects were described in Table 1. Especially CP patients presented with muscle power weakness, spasticity and range of motion in parts of joint contracture.
Total 12 subjects underwent DBS surgery in GPi. All subjects were assessed before the surgery. Then therapeutic outcomes were assessed after 1 month and every 6 months over two years following DBS treatment. Clinical assessment of dystonia was based on the Burke-Fahn-Marsden dystonia rating scale (DRS) which consisted of dystonia movement scale and disability scale.
The degree of dystonia movement of the patients was assessed to investigate the objective effect of DBS. The dystonia movement scale evaluates dystonia in nine body areas, including eyes, mouth, speech and swallowing, neck, trunk, and right and left arms and legs. The arms and legs are given one rating each, without distinguishing proximal and distal elements. Severity ratings range from 0 (no dystonia) to 4 (severe dystonia) for each of the nine body areas. The provoking factor rating assesses the situation in which the dystonia occurs and ranges from 0 (no dystonia) to 4 (dystonia at rest). The scores for eyes, mouth, and neck are each multiplied by 0.5 before being entered into the calculation of the total score. The total score of the dystonia movement scale is the sum of the products of the provoking, severity and weighting factors. The full total score for the dystonia movement scale is 120. The higher the severity of the degree of dystonia was the higher was the score given.
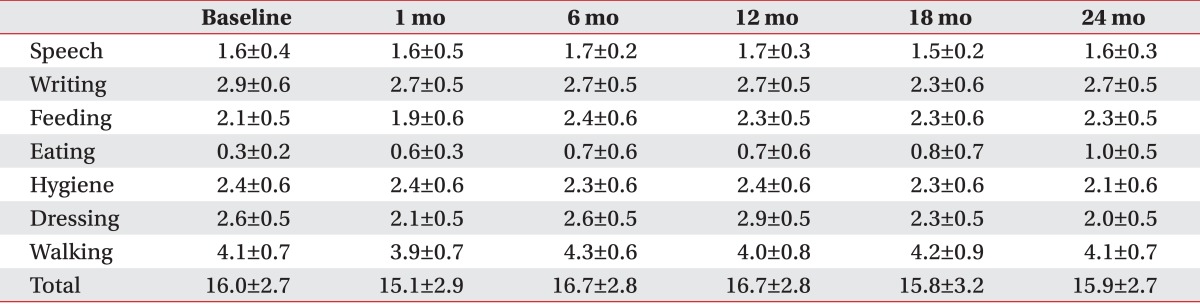
The degree of functional impairment of the patients was assessed to investigate the functional effect of DBS. The disability scale has six categories. Speech, writing, feeding, eating, hygiene, and dressing were each scored from 0 to 4 and a walking subscale was scored from 0 to 6. The higher the degree of disability was the higher the score given was. The scores summed up to a total of 30.
The degree of subjective satisfaction was assessed to investigate the subjective effect of DBS. The subjective satisfaction was scored from 0 to 10, with 10 indicating the highest satisfaction and 0 indicating no satisfaction. Namely, the higher the subjective satisfaction was the higher the score given was.
Scores on the dystonia movement scale and the disability scale were analyzed using repeated measure ANOVA to compare between-group changes and in-group changes. The scores were also compared between primary dystonia patients and CP patients in each assessment using Mann-Whitney U test. In addition, the percentages of the dystonia movement scale, and the disability scale, (%), and the satisfaction scale (score) were compared between the groups using the Mann-Whitney U test 1 year and 2 years after DBS.
Two groups of primary dystonia patients and CP patients showed significant decreases on the dystonia movement scale in the time following DBS (F=15.008, p<0.001). Briefly, the dystonia movement scales of the patients with primary dystonia decreased from 54.8±10.2 to 29.4±9.5 after 1 month, further to 10.8±4.6 after 1 year and to 4.5±3.3 after 2 years of DBS (Fig. 1A). However, the reduction of dystonia movement in adults with CP was not sustained after 1 year, even though their dystonia movement scores showed a similar trend of decrease until 1 year after DBS. Namely, the dystonia movement scores of the CP patients decreased from 66.7±8.2 to 33.1±6.1 after 1 year, but the mean score was slightly increased to 40.0±8.9 until 2 years after DBS (Fig. 1A).
Comparing the dystonia movement scores against those of the CP patients at each assessment 1 month after DBS and at 6 month interval until 2 years after DBS, the scores of the patients with primary dystonia showed a significant reduction after 6 months of DBS treatment until 2 years after DBS (p=0.030 at 6 months, p=0.018 at 12 months, p=0.018 at 18 months, p=0.010 at 24 months after DBS) (Fig. 1A). Consequently, the reduction of dystonia movement score was significantly different over time between the two groups (F=7.344, p=0.022).
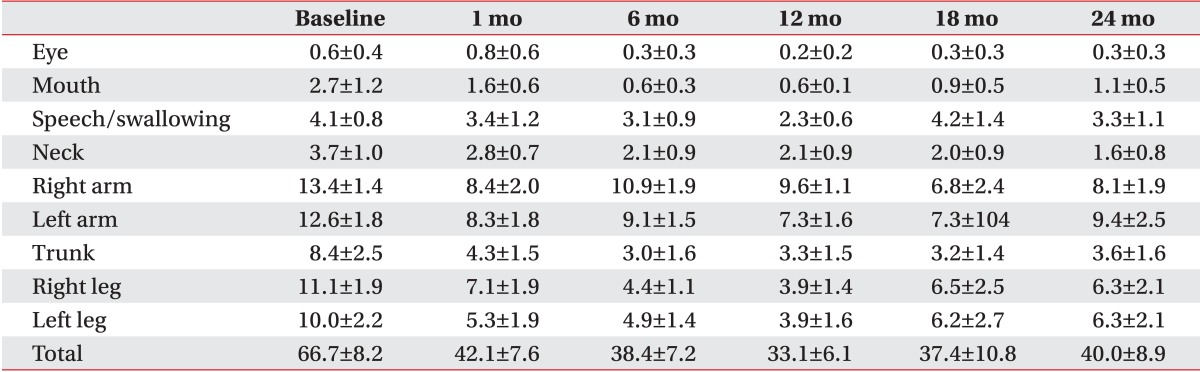
All patients with primary dystonia (100%) and six of seven CP patients (85.7%) showed improvement when we investigated the changes in the dystonia movement scale of individual subjects (Table 2). All nine body area subscores of patients with primary dystonia had a tendency to steadily decrease until 2 years after DBS when we also assessed subscores of dystonia movement scale following DBS (Table 3). However, the CP patients with dystonia showed an increase in the subscores for speech and swallowing after 1 year following DBS (Table 4).
The patients with primary dystonia showed a significant decrease on the disability scale after DBS, whereas that of CP patients with dystonia did not change in 2 years, resulting in a significant difference between two groups (F=9.0, p=0.013). Briefly, the disability scale of the patients with primary dystonia decreased from 11.0±2.3 to 8.4±2.0 after 1 month, further to 4.8±1.0 after 1 year and to 2.6±1.2 at 2 years after DBS (Fig. 1B). However, the disability scale in the adults with CP was maintained in a range from 16.0±2.7 to 15.9±2.7 during the 2 years following DBS (Fig. 1B).
Comparing the disability scales between both groups at each assessment, 1 month after DBS and every 6 months until 2 years after DBS, the patients with primary dystonia showed a significant reduction after 6 months until 2 years after DBS treatment (p=0.003 at 6 months, p=0.003 at 12 months, p=0.005 at 18 months, p=0.003 at 24 months after DBS) (Fig. 1B).
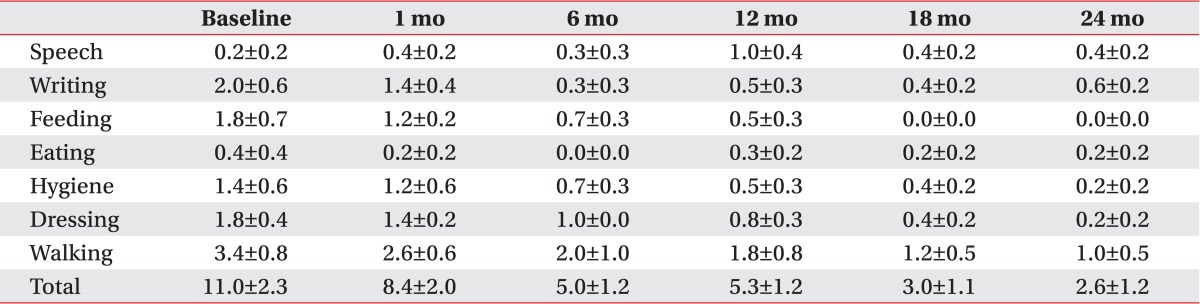
All patients with primary dystonia showed an improvement when we investigated the changes on the disability scale of the individual subjects. However, three of seven CP patients (42.9%) did not show any improvement (Table 2). The assessment of the subscores of disability scale following DBS of the patients with primary dystonia showed a steadily decrease for all subscores except speech in the 2 years after DBS (Table 5). However, the CP patients with dystonia showed no improvement for all subscores; speech, writing, feeding, eating, hygiene, dressing, and walking after DBS (Table 6).
The estimation of the percentage of improvement of the dystonia movement scale relative to the preoperative baseline score for the patients with primary dystonia showed an improvement in dystonia symptoms of 79.5%±6.5% at 1 year after DBS and a further improvement to 92.9%±4.7% at 2 years after DBS (Fig. 2A). On the other hand, the CP patients with dystonia showed an improvement of 46.4%±9.6% in dystonia movement at 1 year after DBS, but the percentage of improvement rather decreased to 39.2%±11.2% at 2 years after DBS (Fig. 2A). Comparing the percentage of improvement with that of the CP patients at 1 year and at 2 years after DBS, the patients with primary dystonia showed a significant improvement at 1 year (p=0.018) and at 2 years (p=0.005) after DBS (Fig. 2A).
The estimation of the percentage of improvement of the disability scale for the patients with primary dystonia showed a disability improvement of 46.6%±18.4% at 1 year after DBS and a further improvement to 80.7%±7.8% at 2 years after DBS (Fig. 2B). However, the CP patients with dystonia did not show any disability improvement after DBS treatment (-6.6%±8.1% at 1 year, 0.5%±6.6% at 2 years) (Fig. 2B). Comparing the percentage of improvement with that of the CP patients at 1 year and at 2 years after DBS, the patients with primary dystonia showed an overall improvement at 1 year (p=0.073) and at 2 years (p=0.003) after DBS (Fig. 2A).
The assessment of subjective satisfaction using a 10-point scale showed for primary dystonia a score of 8.3±0.5 at 1 year after DBS, which was increased to 9.2±0.4 after 2 years following DBS (Fig. 3). On the other hand, the CP patients with dystonia showed a score of 5.5±1.0 at 1 year after DBS, but showed a decreased satisfaction scale score of 4.0±1.5 at 2 years after DBS (Fig. 3). Comparing the satisfaction scales with those of the CP patients, the patients with primary dystonia showed a significantly higher subjective satisfaction at 1 year (p=0.048) and at 2 years (p=0.010) after DBS (Fig. 3).
DBS has been effectively used to treat PD, essential tremor and dystonia as well as psychiatric disorders [17,18,19,20,21]. DBS is nowadays used as a treatment for PD patients without response to pharmacologic treatment, since the DBS treatment has been attempted to ameliorate resting tremor, bradykinesia and levodopa-induced dyskinesia in PD patients [22,23]. In addition, DBS in GPi has shown its significant benefit in patients with primary dystonia [11,12]. DBS has been known to control the signal transduction output that travels from basal ganglia to brain stem, thalamus and cerebral cortex to adjust the dystonia movement because abnormal movement and posture in patients with dystonia is thought to arise from problems in the basal ganglia pathway [24].
Previous studies have shown that DBS produces higher therapeutic effect on primary dystonia patients than on secondary dystonia patients [12,25,26]. There was no distinct lesion that seems to cause symptoms in primary dystonia patients, implying that other reversible electrophysiological or neurochemical factors may be responsible for responding to DBS. On the other hand, it has been suggested, the existence of irreversible anatomical lesions in patients with secondary dystonia may lower response to DBS [27].
A previous pilot study demonstrated that bilateral pallidal DBS resulted in a sustained improvement in motor symptoms and functional disability over 1 year [13]. However, this study disclosed no improvement of the disability scale of CP patients for 2 years, whereas there was only a short-term decrease in dystonia movement scale until 6 months to 1 year after DBS. CP patients with dystonia also exhibited disappointing outcomes in dystonia movement, disability and satisfaction compared to patients with primary dystonia in the long-term period until 2 years after DBS treatment. Furthermore, three of total 7 patients with CP (42.9%) did not show any subjective satisfaction at all. The limitation of the improvement of disability and satisfaction score in the CP patients might be caused by other motor related impairments such as muscle power weakness, spasticity, and joint contracture.
As Vidailhet et al. [13] mentioned in their report, DBS in GPi appeared to show no dramatic improvement for a long-term period in CP patients because electrical diffusion to globus pallidus externa (GPe) might have resulted in no effect or worsening dystonia movement symptoms in some subjects of this study. In addition, the presence of the characteristic discrepancy between dystonia movement and functional disability in CP patients after DBS suggests that a change in fixed pattern of abnormal movement and posture from the birth or neonatal period did not alleviate or rather worsened the function itself.
Nevertheless, although improvement in CP patients was less efficient compared with patients with primary dystonia who have shown a dramatic improvement of 92.9%, CP patients have shown a modest improvement of 39.2% on the dystonia movement scale. We also suggest other therapeutic indications of reducing muscle tone and musculoskeletal pain rather than functional improvement in CP patients with severe dystonia.
This study had its limitations as it was not a controlled study and had a limited number of subjects. Therefore, a more detailed study is needed to investigate appropriate candidates and to elucidate therapeutic mechanism of DBS among a large number of patients with secondary dystonia including patients with CP in the future.
In conclusion, whereas dystonia can be significantly reduced by performing bilateral pallidal DBS in patients with primary dystonia, CP patients showed a modest improvement on the dystonia movement scale, but not on the disability scale. Therefore, DBS may be considered with caution as a treatment modality for CP patients with dystonia to reduce the dystonia movement.
ACKNOWLEDGMENTS
This study was supported by grants from the National Research Foundation (NRF-2010-0024334) funded by the Ministry of Education, Science and Technology, Republic of Korea.
References
1. Bhidayasiri R. Dystonia: genetics and treatment update. Neurologist. 2006; 12:74–85. PMID: 16534444.
2. Fahn S. Concept and classification of dystonia. Adv Neurol. 1988; 50:1–8. PMID: 3041755.
3. Hartmann A, Pogarell O, Oertel WH. Secondary dystonias. J Neurol. 1998; 245:511–518. PMID: 9747914.

4. Jankovic J. Dystonia: medical therapy and botulinum toxin. Adv Neurol. 2004; 94:275–286. PMID: 14509685.
5. Gilderberg PL, Tasker RR. Textbook of stereotactic and functional neurosurgery. 1st ed. New York: Springer;1998.
6. Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics. 2008; 5:320–330. PMID: 18394573.

7. DeLong M, Wichmann T. Deep brain stimulation for movement and other neurologic disorders. Ann N Y Acad Sci. 2012; 1265:1–8. PMID: 22823512.

8. Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollak P. Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord. 1998; 13(Suppl 3):119–125. PMID: 9827607.

9. Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson's disease. Mov Disord. 2002; 17(Suppl 3):S145–S149. PMID: 11948769.

10. Olanow CW, Brin MF, Obeso JA. The role of deep brain stimulation as a surgical treatment for Parkinson's disease. Neurology. 2000; 55(12 Suppl 6):S60–S66. PMID: 11188977.
11. Vercueil L, Krack P, Pollak P. Results of deep brain stimulation for dystonia: a critical reappraisal. Mov Disord. 2002; 17(Suppl 3):S89–S93. PMID: 11948761.

12. Eltahawy HA, Saint-Cyr J, Giladi N, Lang AE, Lozano AM. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery. 2004; 54:613–619. PMID: 15028135.

13. Vidailhet M, Yelnik J, Lagrange C, Fraix V, Grabli D, Thobois S, et al. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study. Lancet Neurol. 2009; 8:709–717. PMID: 19576854.

14. Pretto TE, Dalvi A, Kang UJ, Penn RD. A prospective blinded evaluation of deep brain stimulation for the treatment of secondary dystonia and primary torticollis syndromes. J Neurosurg. 2008; 109:405–409. PMID: 18759568.

15. Zhang JG, Zhang K, Wang ZC, Ge M, Ma Y. Deep brain stimulation in the treatment of secondary dystonia. Chin Med J (Engl). 2006; 119:2069–2074. PMID: 17199958.

16. Katsakiori PF, Kefalopoulou Z, Markaki E, Paschali A, Ellul J, Kagadis GC, et al. Deep brain stimulation for secondary dystonia: results in 8 patients. Acta Neurochir (Wien). 2009; 151:473–478. PMID: 19322514.

17. Hardesty DE, Sackeim HA. Deep brain stimulation in movement and psychiatric disorders. Biol Psychiatry. 2007; 61:831–835. PMID: 17126303.

18. Kopell BH, Greenberg B, Rezai AR. Deep brain stimulation for psychiatric disorders. J Clin Neurophysiol. 2004; 21:51–67. PMID: 15097294.

19. Yu H, Neimat JS. The treatment of movement disorders by deep brain stimulation. Neurotherapeutics. 2008; 5:26–36. PMID: 18164481.

20. Krauss JK, Yianni J, Loher TJ, Aziz TZ. Deep brain stimulation for dystonia. J Clin Neurophysiol. 2004; 21:18–30. PMID: 15097291.

21. Tagliati M, Shils J, Sun C, Alterman R. Deep brain stimulation for dystonia. Expert Rev Med Devices. 2004; 1:33–41. PMID: 16293008.

22. Krack P, Pollak P, Limousin P, Benazzouz A, Deuschl G, Benabid AL. From off-period dystonia to peak-dose chorea: the clinical spectrum of varying subthalamic nucleus activity. Brain. 1999; 122(Pt 6):1133–1146. PMID: 10356065.

23. Kupsch A, Earl C. Neurosurgical interventions in the treatment of idiopathic Parkinson disease: neurostimulation and neural implantation. J Mol Med (Berl). 1999; 77:178–184. PMID: 9930959.

24. Tisch S, Rothwell JC, Limousin P, Hariz MI, Corcos DM. The physiological effects of pallidal deep brain stimulation in dystonia. IEEE Trans Neural Syst Rehabil Eng. 2007; 15:166–172. PMID: 17601185.

25. Kim JP, Chang WS, Park YS, Chang JW. Bilateral globus pallidus internus deep brain stimulation for DYT1+ generalized dystonia with previously received bilateral thalamotomy and unilateral pallidotomy. Stereotact Funct Neurosurg. 2011; 89:205–209. PMID: 21597310.

26. Alcindor D, Oh MY, Baser S, Angle C, Cheng BC, Whiting D. Stimulation of the globus pallidus internus in a patient with DYT1-positive primary generalized dystonia: a 10-year follow-up. Neurosurg Focus. 2010; 29:E16. PMID: 20672918.

27. Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry. 2010; 81:1383–1389. PMID: 20841370.

Fig. 1
Changes in dystonia rating scale over time up to 2 years after deep brain stimulation (DBS). (A) Two groups of primary dystonia and cerebral palsy (CP) patients showed a significant decrease over time following DBS on the dystonia movement scale. However, the mean score in CP patients was rather slightly increased after 1 year following DBS. Consequently, the reduction of dystonia movement score was significantly different over time between two groups. (B) Patients with primary dystonia showed a significant decrease over time on the disability scale following DBS, whereas that of CP patients with dystonia did not change up to 2 years, resulting in a significant difference between two groups. *p<0.05.
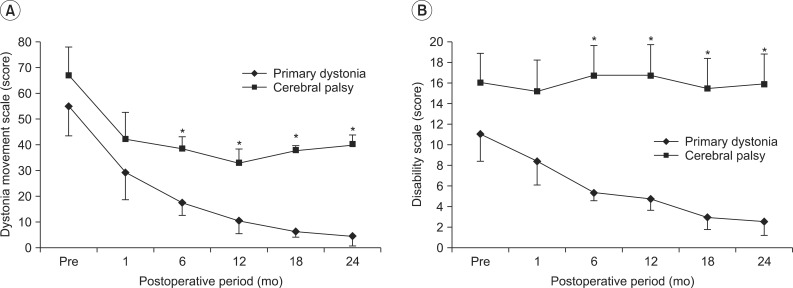
Fig. 2
Percentages of improvement on the dystonia rating scale at 1 year and 2 years after deep brain stimulation (DBS). (A) When the percentages of improvement relative to the preoperative score were estimated on the dystonia movement scale, cerebral palsy (CP) patients showed an improvement in dystonia movement after 1 year following DBS, but the improvement (%) rather decreased after 2 years following DBS, whereas patients with primary dystonia showed improvement in dystonia symptoms up to 2 years following DBS. Consequently, patients with primary dystonia showed a significant improvement at 1 year and 2 years following DBS, compared with CP patients (p<0.05). (B) When the percentages of improvement (%) were estimated on the disability scale, patients with primary dystonia showed an improvement in disability at 1 year after DBS and improved further at 2 years following DBS. However, CP patients with dystonia did not show any improvement in disability after DBS treatment, resulting in a significant difference between two groups at 2 years following DBS (p<0.05). *p<0.05.
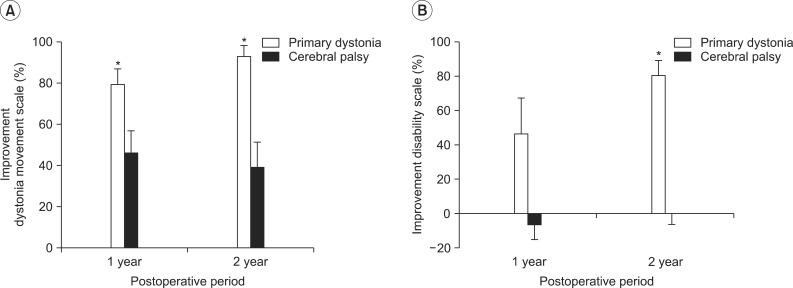
Fig. 3
Subjective satisfaction scales at 1 year and 2 years after deep brain stimulation (DBS). When the degree of subjective satisfaction was assessed by a 10 score scale, cerebral palsy (CP) patients with dystonia showed a score of 5.5±1.0 at 1 year after DBS, but showed a decrease in the satisfaction score of 4.0±1.5 after 2 years following DBS, whereas patients with primary dystonia showed a score of 8.3±0.5 at 1 year after DBS and they further increased to a score of 9.2±0.4 after 2 years following DBS. Consequently, patients with primary dystonia showed a significantly higher subjective satisfaction at 1 year and 2 years following DBS (p<0.05). *p<0.05.
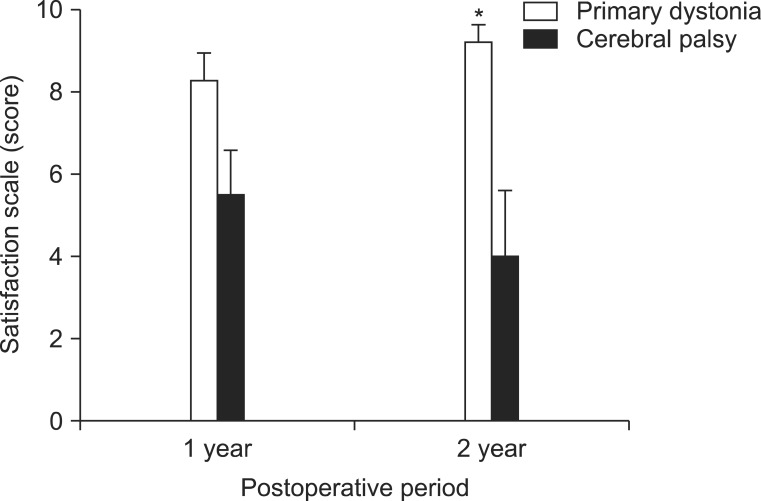
Table 2
Changes in on the dystonia rating scale at baseline and 2 years after deep brain stimulation
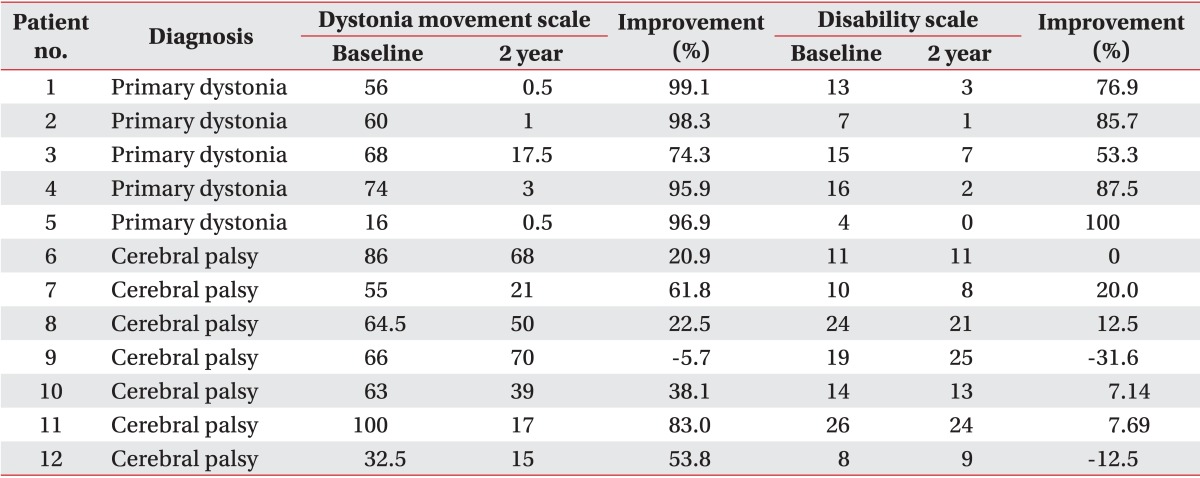
Table 3
Changes in subscores of the dystonia movement scale up to 2 years after deep brain stimulation in patients with primary dystonia
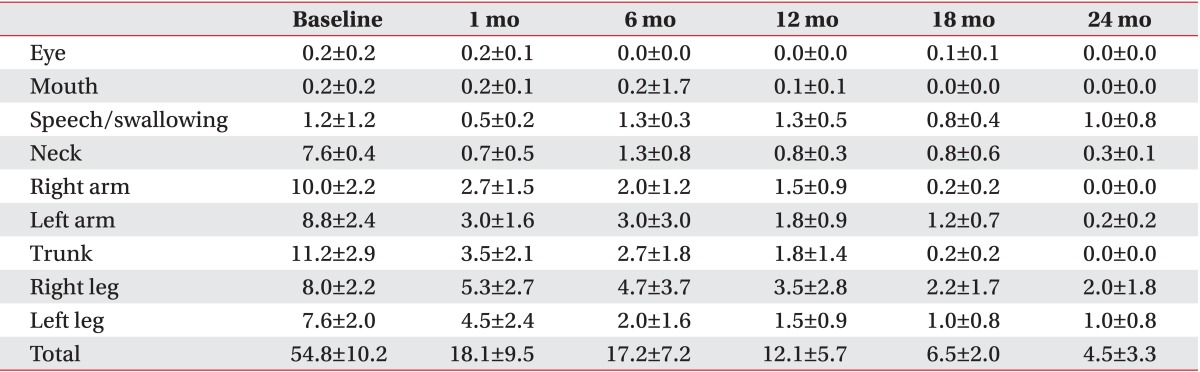
Table 4
Changes in subscores of the dystonia movement scale up to 2 years after deep brain stimulation in adults with cerebral palsy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download