INTRODUCTION
Bruxism is a diurnal or nocturnal parafunctional activity that includes clenching, bracing, gnashing, and grinding of the teeth. Its prevalence is reported to be 20% among the adult population [
1]. Previous studies have pointed out alterations in central neurotransmission, particularly dopaminergic neurotransmission, as the main cause of bruxism. Recent studies suggest that hypersensitivity of the dopamine receptors is associated with bruxism [
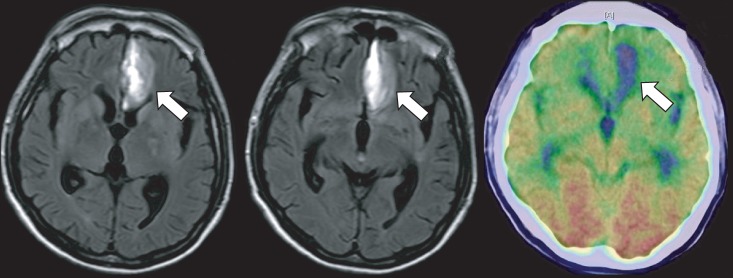
2]. We report two patients with diurnal Medicinebruxism in whom bilateral frontal lobe hypoperfusion resulted from hemorrhagic stroke or traumatic brain injury. These patients' bruxism was refractory to bromocriptine but responded to metoclopramide therapy. These cases suggest that blockade of the hypersensitive presynaptic dopaminergic receptor can reduce bruxism.
Go to :

DISCUSSION
The pathogenesis of bruxism continues to be a matter of scientific debate, and different theories have been proposed over the years. Previous studies revealed that individuals with sleep bruxism have higher levels of urinary catecholamines including dopamine [
3]. Bruxism may develop after exposure to haloperidol or selective serotonin reuptake inhibitors (SSRIs) [
3], or in untreated schizophrenia [
4]. These studies suggested that the central dopaminergic system may be of some importance in explaining the origin of bruxism.
The central role of the frontal lobe in bruxism is supported by a previous study [
5]. Our patients had a bilateral frontal lobe injury that resulted from a hemorrhagic stroke or traumatic brain injury. Although dopamine receptors are widely distributed in the brain, different areas have different receptor types and densities, presumably reflecting different functional roles in different areas. The mesocortical pathway, which is a neural pathway that connects the ventral tegmentum to the frontal cortex, modulates the motor output of the prefrontal cortex by maintaining an inhibitory signal [
6].
The acute (short-term) use of L-dopa, a dopamine precursor and a precursor of bromocriptine, a dopamine receptor agonist, inhibits bruxism activity in controlled polysomnographical studies [
7]. Meanwhile, medications that exert an indirect influence on the dopaminergic system, like selective serotonin reuptake inhibitors or dopaminoceptor antagonists, are known to induce bruxism [
2]. These studies suggest that bruxism may be induced by diminution of dopaminergic transmission at the prefrontal cortex.
But interestingly, our patients showed another interaction of neurochemistry in diurnal bruxism. Our patients were refractory to bromocriptine, but responded well to metoclopramide, a selective dopamine receptor antagonist. This can be explained by the preferential action of metoclopramide on presynaptic dopamine receptors. While a high dose of metoclopramide is known to block postsynaptic dopamine receptors, a low dose of metoclopramide is more potent in blocking dopamine receptors of the dopamine nerve terminals (presynaptic dopamine receptors) [
8]. It results in a facilitated release of dopamine without a blockade of the postsynaptic dopamine receptors [
8]. Therefore, a selective blockade of the hypersensitive presynaptic dopamine receptors by low-dose metoclopramide administration recovers the dopaminergic flow and attenuates bruxism. On the other hand, L-dopa and bromocriptine activate both the presynaptic and postsynaptic dopamine receptors with a net effect, resulting in no changes in dopaminergic innervations [
2].
Chen et al. [
2] reported a subjective reduction of bruxism activity observed in three patients receiving dopamine receptor antagonist medication. The patients had left frontal hypoperfusion on imaging and showed no effect to the previous administration of either L-dopa or bromocriptine. The reduction of bruxism was associated with a reperfusion of the prefrontal cortex during wake-time. It has been suggested that in patients with both awake and sleep bruxism, a different mechanism may operate in the genesis of bruxism [
2].
Drug use, such as SSRIs, methylenedioxymethamphetamine, methylenedioxyamphetamine, methylphenidate, and other amphetamines may cause bruxism [
9]. The concurrent administration of anticholinergic drugs, pramlintide, for example, is known to decrease the effectiveness of metoclopramide, and such medications were not administered in our cases [
10]. There was also no administration of drugs that may interfere with the actions of levodopa, such as haloperidol, loxapine, phenothiazines, in our cases.
In an animal study, 0.3 mg/kg of metoclopramide given intraperitoneally facilitated the release of dopamine. A dose ten times higher did not block presynaptic dopamine receptors but seemed to block postsynaptic dopamine receptors, judging from the inhibition of the apomorphine-induced turning [
8]. The proper metoclopramide dosage for bruxism might be the usual adult dose for gastroparesis, depending upon the symptoms being treated and the clinical response. The usual dose of metoclopramide for treating gastroparesis is 10 to 15 mg up to 3 times a day orally.
In this study, we report two patients with bruxism in whom bilateral frontal lobe lesions resulted from an acquired brain injury. These patients' bruxism was refractory to bromocriptine but responded to low-dose metoclopramide therapy. Our cases indicate that administering a low dose of metoclopramide is possibly a sound method for treating bruxism in a brain injury patient with frontal lobe hypoperfusion on a PET image. Further study is needed under blind and controlled conditions using various tools to characterize the symptoms of bruxism, such as an EMG and audio/video or polysomnographic assessments.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download