DISCUSSION
Many attempts have been made to provide compensatory sensory stimulation including transcutaneous electrical nerve stimulation [
8], functional electrical stimulation [
9], electromyographic feedback [
10] or force feedback training to improve standing balance [
11]. Unfortunately, none of the physiotherapies are superior for promoting balance recovery in stroke patients [
12]. Thus, a novel rehabilitation approach is needed.
Here, we targeted the misperception of verticality, a major cause of impaired standing balance. The correct perception of verticality is formed by integrating adequate visual, vestibular, and somatosensory input [
13]. Based on these afferent inputs, internal sensory integration process construct perception of verticality, which is normally parallel to the earth's vertical axis [
13,
14]. It can be evaluated through different modalities, such as visual, haptic, and postural [
13,
15]. Among these, we targeted visual and haptic verticals. Separate 867 exist for the perception of VV and HV [
4,
16]. VV perception is mainly based on visual and vestibular input from peripheral organs [
4]. In contrast, HV perception originates from peripheral somatosensory input [
4,
16]. There is often dissociation between the two vertical stimulations [
4,
16]. Nevertheless, post-stroke patients could have a problem with afferent input or integration disorders of different sensory modalities that may lead to the misperception of verticality [
14]. In our study, all subjects had somatosensory loss on the contralesional side. We postulated that our patients might have a misperception of verticality based on the abovementioned reasons; thus, we tried to reduce this bias through the appropriate manipulation, such as correct vertical stimulation. In general, to assess the visual vertical, the subject was instructed to adjust a luminous rod in the vertical direction in the darkness [
17,
18]. The subject was asked to set a rotating bar to the vertical direction by using his/her tactile sense in the darkness to evaluate the haptic vertical [
17,
18]. Based on these evaluation methods, we designed our study to provide the correct VV and HV stimulations; we analyzed their effects through posturography. The typical characteristics of unstable standing in post-stroke patients are weight-bearing asymmetry with more weight placed on the nonparetic leg and a large postural sway [
19]. Considering these characteristics, we focused on two aspects of standing balance: steadiness and symmetry. Steadiness is the ability to maintain a standing posture with minimal movement (i.e., sway) [
20]. It is generally assessed as the amount of COP displacement [
21,
22]. COP sway length, area, and velocity are commonly used to assess postural control [
21] and were used in the present study as parameters of COP displacement. However, symmetry is a term used to describe an equal weight distribution between the two feet in a standing position [
20]. It can be assessed by measuring the left/right pressure difference. Achieving weight-bearing symmetry with minimal sway has been viewed as a primary goal of rehabilitation [
23].
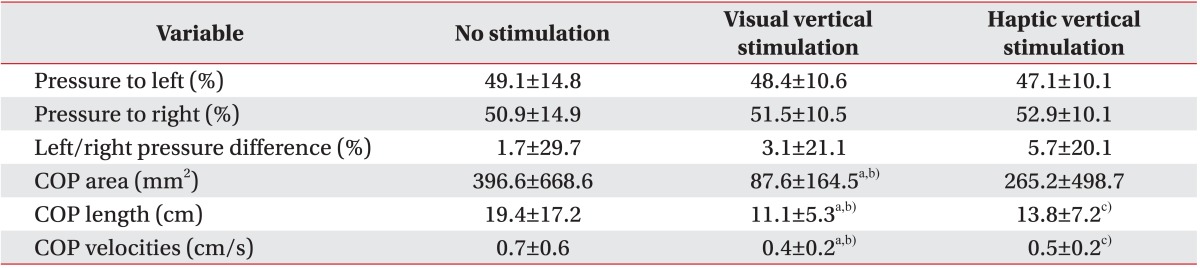
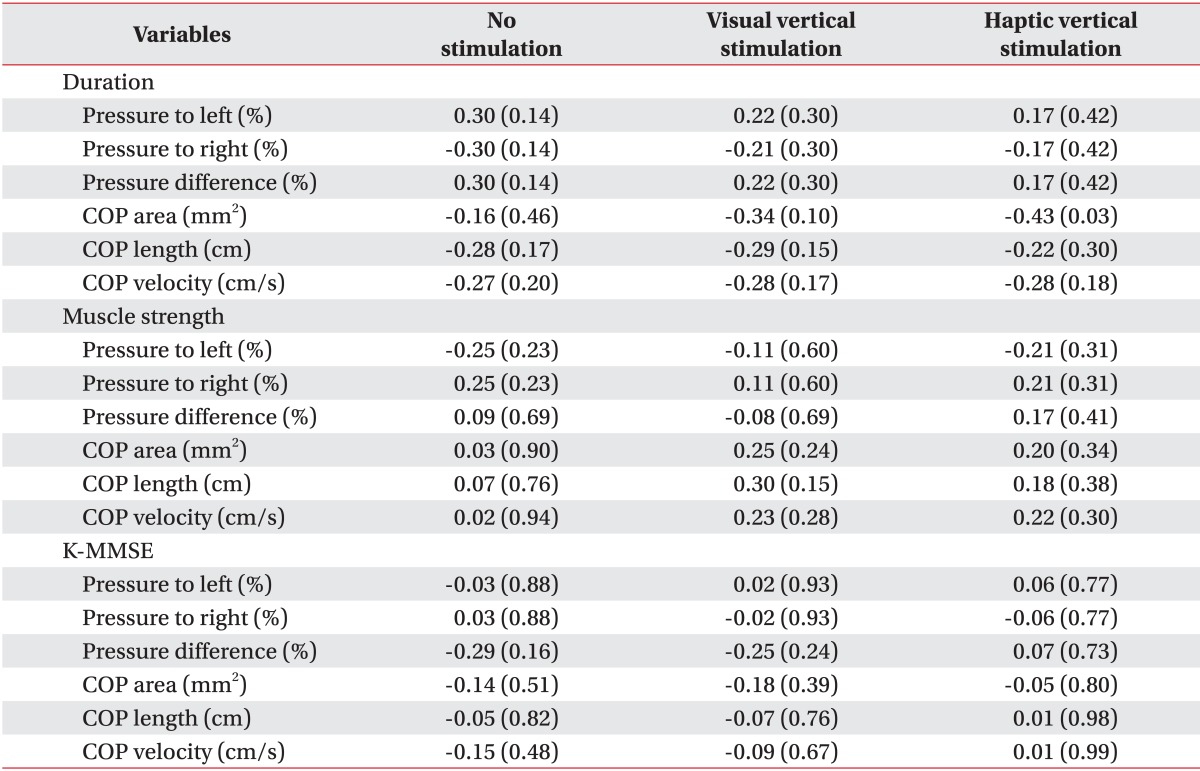
In our study, the COP sway parameters were reduced in both the VV and the HV stimulation conditions. That is, subjects were more stable when gravitational cues were provided. It has been proposed that gravitational cues are favored during orientation processing because they specify the gravitational vertical, which is used as a reference [
24]. Although the mechanism underlying this effect has not been characterized, it may be due to adapting the excitability of the integrative centers and the brain circuits implicated in balance control [
25]. Additionally, a greater reduction in COP parameters was observed in the VV stimulation condition than the HV stimulation condition. When surrounded by many visual, vestibular, and somatosensory stimuli, stroke patients may favor one system over another to control standing balance [
26]. It has also been reported that post-stroke patients depend more on visual information for balance control than healthy age-matched groups [
23]. It has long been known that vision is a major determinant of balance control [
18] and excessive reliance on visual input may be a learned compensatory response that occurs over time, particularly in patients who have a somatosensory impairment [
26]. Our patients likely compensated for their impaired balance using surrounding visual information rather than information from other sensory modalities. This would explain the observation of greater improvements in the VV stimulation condition. However, no reports have indicated that visual stimulation is superior to haptic stimulation for standing balance. Additional research is needed to compare the effect of different sensory modalities, which often have dissociable effects, on vertical perception [
13,
16,
27].
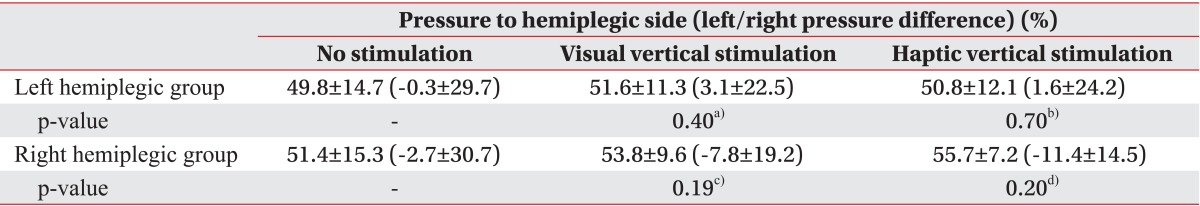
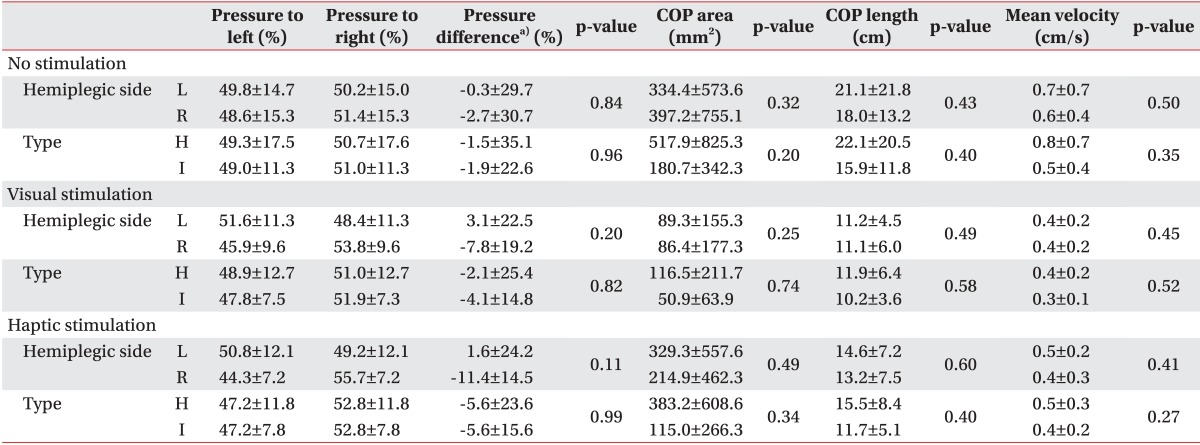
Interestingly, unlike the left hemiplegic patients, the right hemiplegic patients bore more weight on their weaker side. This result is inconsistent with the general concept that more weight is loaded on the nonparetic side in hemiplegic patients [
19]. However, according to one report, more weight-bearing on the paretic side is observed in about 12% of stroke patients [
28]. In that study, no individual determinants of the weight-bearing side were found. Similarly, we investigated sex, age, stroke duration, stroke type, cognition, and muscle power and found no individual determinants. This may be due to sustained pushing behavior or a compensatory strategy learned in a rehabilitation program [
28]. More weight on the paretic side has an advantage in that it allows for the rapid step of the intact limb in case of instability [
28]. Additionally, we found that more weight was shifted to the paretic side and weight-bearing asymmetry deteriorated under the VV and HV stimulation conditions, although significant differences were not observed. However, weight-bearing asymmetry may not be related with COP parameters [
29] and it is not clear whether weight-bearing asymmetry is associated with postural instability [
30]. Rather it is effective compensatory method to restore standing balance after stroke [
31]. In our study, COP parameters were improved despite more weight-bearing to the paretic side and exaggerated weight-bearing asymmetry. This may have been due to increasing the somatosensory input and enhancing kinetic contribution from paretic limb. Our patients had sufficient power in their paretic limb to tolerate more weight.
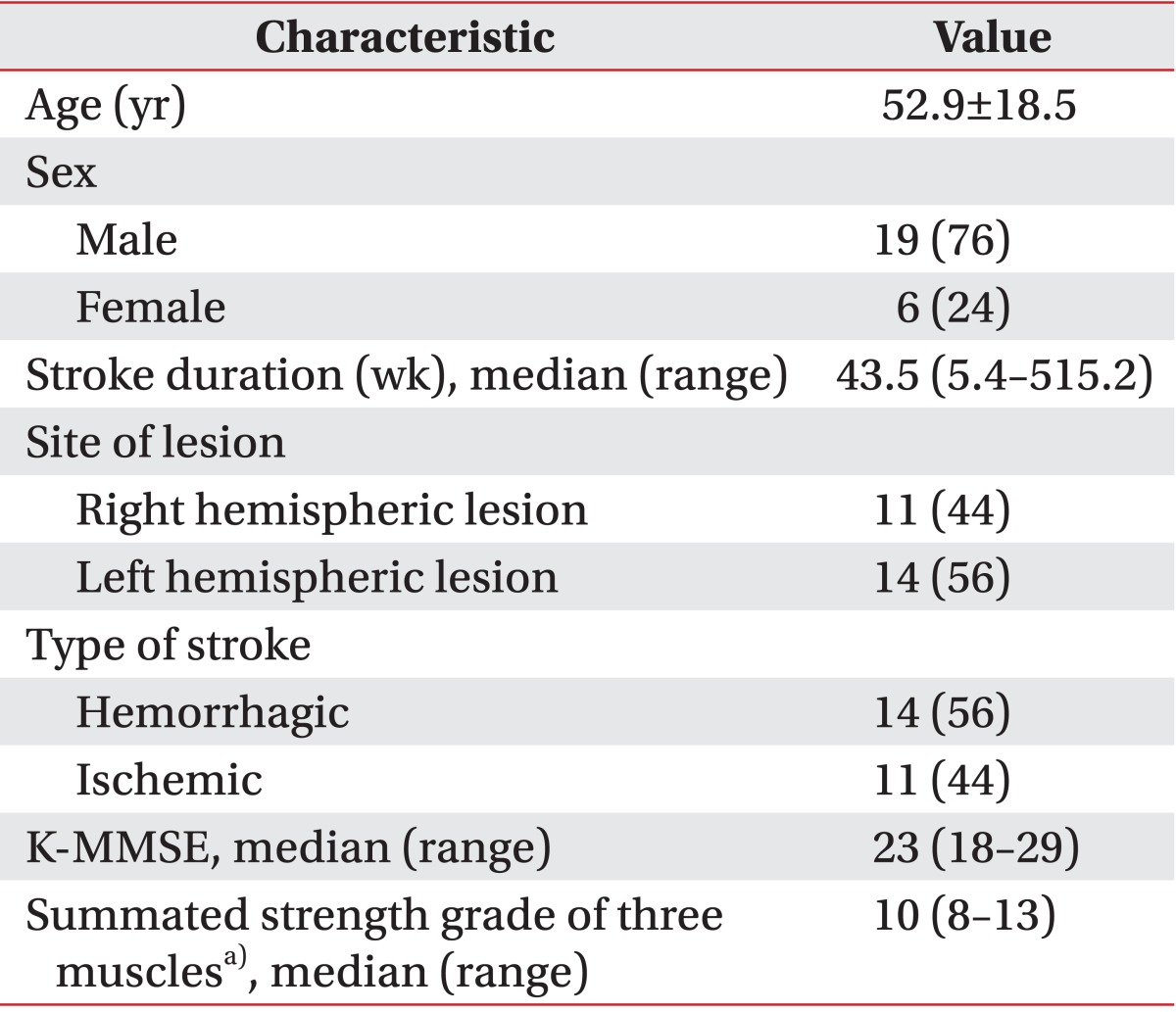
There are some limitations in interpreting and verifying the results of this intervention. First, the number of subjects in the study was too small to generalize the results to a wider group of people with stroke. Second, we did not assess the existence of visual and haptic vertical misperception of subjects. Instead, we assumed that none of the subjects had a correct perception of verticality because they all had contralesional somatosensory deficits. Although the degree of hypoesthesia can be a factor that affects verticality perception, we only determined whether the patients had hypoesthesia. Third, when alternating between the three intervention approaches (i.e., no stimulation, VV stimulation, and HV stimulation), the carryover effect from one approach to another was not strictly controlled. Fourth, no statistical analysis was conducted for the interaction between brain lesion and stimulation. The central vestibular pathways (e.g., the brainstem, thalamus, cortex) of both cerebral hemisphere, sensory pathways (e.g., the thalamus, sensory cortex), and regions implicated in visuospatial process (e.g., the parietal cortex) are known to be associated with verticality perception [
10]. Because the sample was small and the patients' brain lesions were heterogeneous, we could not determine whether an interaction existed. Fifth, two types of information were given to the patients when the HV stimulation was applied. One was related to the provision of a vertically fixed reference point in space and the other was related to the information provided by transient mechanical forces developed between the hand and the contact surface. We intended to provide the first type of information; the latter type of information was unintended. To minimize this effect, subjects were instructed to gently touch the vertical rod using their finger tips. Unfortunately, we cannot rule out an effect of the latter type of information.
In conclusion, we focused on the effect of direct visual and haptic vertical stimulation on standing balance of stroke patients. Left/right pressure difference, namely, weight-bearing symmetry got worse with either stimulation. However, both stimulations reduced COP displacement parameters. In other words, standing balance, particularly steadiness, improved by providing the correct vertical stimulation. Furthermore, this effect was more prominent in VV stimulation than HV stimulation. Thus, this approach may provide the basis for an effective rehabilitation program for post-stroke patients who suffer from standing balance impairment.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download