Abstract
Guillain-Barre syndrome (GBS) and syringomyelia are diseases of different entities. GBS is an acute post-infectious autoimmune disease which is mediated by autoantibodies against the myelin of peripheral nerves. Syringomyelia is a chronic disease characterized by a cavity extending longitudinally inside the spinal cord. A 67-year-old man is being hospitalized due to severe numbness and ascending weakness in all limbs. On neurological examination, the motor power of all limbs are decreased and show absence of deep tendon reflexes (DTRs). The patient is being diagnosed with GBS on the basis of the acute clinical course, nerve conduction studies of segmental demyelinating polyneuropathy, and a finding of albuminocytologic dissociation in the cerebrospinal fluid. The patient is presented with a new set of symptoms thereafter, which composes of sensory changes in the upper extremities, the urinary dysfunction including frequency and residual urine, spastic bilateral lower extremities, and increased reflexes of the knee and the biceps at follow-up examinations. The spinal magnetic resonance imaging in the sagittal section revealed a syrinx cavity between the fifth cervical and the first thoracic vertebral segment in the cord. The somatosensory evoked potential show sensory pathway defects between both the brachial plexus and the brain stem. Thus, this patient is being diagnosed with both GBS and syringomyelia. We report a case of symptomatic syringomyelia coexisting with GBS. Since the GBS is presented with a progressive muscle weakness and reduced DTRs, the muscle weakness and stiffness in the extremities suggests a concurrent syringomyelia might be easily overlooked.
Guillain-Barre syndrome (GBS) and syringomyelia are diseases of different entities. GBS is an acute post-infectious autoimmune disease mediated by the autoantibodies against the myelin of the peripheral nerves. It is currently being observed worldwide with an incidence between 0.6 and 4 cases per 100,000 even though its incidence depends on the region. It has been associated with antecedent bacterial and viral infections, administration of certain vaccinations, surgery, trauma, and other systemic illnesses [1]. The major clinical manifestation is an ascendant symmetrical weakness with reduced or absent deep tendon reflexes (DTRs). GBS may be presented with an acute onset of progressive limb weaknesses and sensory disturbances in relatively symmetrical manners [2] with minimal sensory losses and cranial nerve palsies.
Syringomyelia is a chronic disease characterized by a cavity extending longitudinally inside the spinal cord [3], and many of the cases are considered as incidental findings. The exact pathogenesis and development of syringomyelia are uncertain [4,5]. Syringomyelia is multifactorial and is associated with several conditions, most notably are the Chiari 1 and 2 malformations, spinal cord tumors, infection, and trauma [6,7]. The most prominent signs include a lack of sensation below the syringomyelia level of the cavity, often in the pattern of a cape or shawl. This is usually followed by muscle weaknesses, a loss of pain and temperature sensations in the upper extremities and spastic paralysis, and with contractions or shortening of the muscles. The signs and symptoms of syringomyelia tend to develop slowly but could also occur abruptly.
We now report a case of symptomatic syringomyelia coexisting with GBS.
A 67-year-old man was hospitalized in our department on August 27, 2010, due to a sudden onset of weakness and numbness of his feet and fingertips. He had no medical history for diabetes mellitus, hypertension, immunization, and other special syndromes except for upper respiratory infection (URI) about 1 week ago. Moreover, he had not experienced any difficulties when performing daily activities, such as walking, self-caring, and house chores. Due to the lack of severity for his URI symptoms which consisted of rhinorrhea, cough, and myalgia, his medications were not consumed. On physical examination, the patient's vital signs were normal. A neurological examination revealed quadriplegia showing fair grade on manual muscle test (MMT) for all limbs, and superficial and deep sensory disturbances for all limbs with absent DTRs. His weakness and numbness were not exactly matched with the dermatome of the limbs. Upper motor neuron signs were not detected in all limbs. In addition, there were no abnormal intracranial findings on his brain computed tomography scans.
After two weeks from his initial presentation, the patient experienced progressive muscle weaknesses, which corresponded to poor grade at upper extremities and grade traces at both lower extremities on MMT. The electrophysiological studies were carried out. The sensory nerve conduction study (NCS) showed relatively normative findings. However, the motor NCS showed decreased amplitude and a partial motor conduction block at the right median nerves and at both the common peroneal and posterior tibial nerves. The motor NCS also showed a prolonged F wave and an absent H-reflex. The needle electromyography showed decreased recruitment and active denervations at the upper and the lower distal muscles. These electrophysiological findings were suggestive of demyelinating polyneuropathy.
To make confirmative diagnosis, a cerebrospinal fluid (CSF) study-via a lumbar spinal puncture-was also performed. The CSF findings showed albuminocytological dissociation. The cumulative findings consisted of the patient's history of preceding infection, neurological examination, electrophysiological studies, and the CSF analysis which met the diagnostic criteria for GBS. The patient also experienced urinary dysfunctions during the initiation of voiding phase in addition to the loss of sensation on bladder fullness. Therefore, he required clean intermittent catheterization. The serum prostate specific antigen level showed mild elevations and a transrectal ultrasonography (TRUS) of the prostate was consistent with benign prostate hyperplasia. Urodynamic studies (UDS) showed an abnormal bladder voiding phase indicated by bladder areflexia. Since he didn't have a history of regular medical consumptions except for immunoglobulin of GBS, thus, medications are less likely to be culprits for his urinary symptoms. The urinary dysfunction in GBS has rarely been reported but there exists few cases of GBS coexisting with bladder areflexia. Therefore, we initially assumed that his bladder areflexia was related to GBS. The motor power of all limbs had slightly improved after a course of intravenous immunoglobulin (IVIG) injection for five days (at a dose of 235 mg per day), but the patient was unable to walk independently. On September 28, 2010, he was transferred to a local rehabilitation facility.
One month later, the patient appeared again at our department. This time, he was found to have new myelopathic signs through examinations that were evidenced by both hypotonic upper extremities showing fair grade on MMT, urinary dysfunction, and spastic lower extremities of both knee flexors with Modified Ashworth Scale grade 2 at follow-up examinations. Although there were no differences in weakness of upper and lower extremities as compared to previous outcomes, we recommended that the patient be admitted to identify other potential causes. He had been kept for conservative treatments at a local rehabilitation facility for 3 months subsequently while his myelopathic signs had continued.
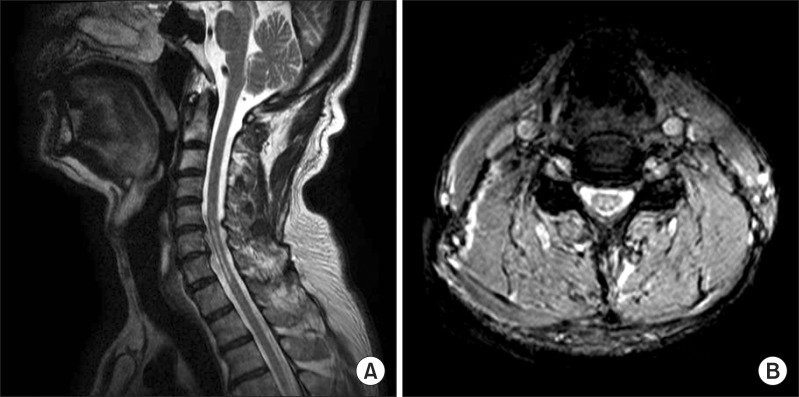
He was hospitalized again in our department on February 11, 2011. He still incurred stiffness in the lower extremities and showed different patterns of urinary dysfunctions which included frequency and urgency with retaining of a large amount of postvoid residual urine. There was bilateral sensory loss of pain and temperature over the dermatomes C5 to T1 with weaknesses of bilateral upper extremities that corresponded to poor on MMT, whereas the deep and superficial perianal sensations were preserved. On neurological examination, he had increased biceps tendon and patellar reflexes. The brain magnetic resonance imaging (MRI) showed no abnormal findings, whereas the MRI scans of the cervical spine revealed a syrinx cavity. This is evident by the hypointense signals in T1-weighted section and the hyperintense signals in T2-weighted section extending between the 5th cervical and the 1st thoracic vertebral segment of the cord (Fig. 1). In addition, mild disc bulging with spinal stenosis at L45/S1 was also found on the whole-spine MRI. The electrophysiological studies showed improvements when compared to the previous study, but still had slow nerve conduction velocities and partial motor conduction blockades. An additional neurological workups consisting of bulbocavernosus (S2-4) reflexes (BCRs), somatosensory evoked potential (SEP) and motor evoked potential were performed. The BCRs were intact while the median nerve SEP showed a sensory pathway defect between both the brachial plexus and the brain stem. These results corresponded to the syrinx extending between the 5th cervical and the 1st thoracic vertebral segments in the cord. To reveal the cause of the urinary dysfunction, voiding cystourethrogram (VCUG), TRUS and UDS were performed. There were no abnormal findings in urine analysis with culture, VCUG and TRUS. The UDS findings were consistent with overactive bladder, but the detrusor external sphincter dyssynergia could not be confidently ruled out. Therefore, based on the imaging studies, the neurological examinations and the findings indicated areflexic bladder, and the patient was diagnosed with syringomyelia.
It is hard to know the exact timing of the onset of syringomyelia. By the time he was diagnosed with GBS, he had no suspicious symptoms of syringomyelia. However, we suggested that GBS and syringomyelia coexisted given his history, neurological examination, and imaging studies. As a result, he received steroid therapy. After one month of medical treatment with inpatient rehabilitation, his weakness in all limbs had slightly been getting better and thus, needed minimal assistance with mobility. Yet, his urinary symptoms failed to improve significantly. Currently, the patient has been discharged home and has visited our outdoor patient department periodically. The last date of the follow-up visit was May 25, 2012. There have been no noticeable improvements of overall physical functions but noticeable improvements in his urinary symptoms allowed him to urinate independently.
GBS is an acute and immune-mediated disorder of the peripheral nervous system and is also the most frequent cause of post-infectious and progressive neuromuscular paralysis with reduced or absent tendon reflexes. The maximal weakness is reached within 4 weeks, but most patients reach the maximum within 2 or 3 weeks. Despite standard treatment with IVIG or plasma exchange treatment, about 20% of severely affected patients remained unable to walk after 6 months. The prognosis of GBS is difficult to predict for each individual patients because of the substantial variations in outcomes [8]. However, the peroneal nerve conduction block in nerve conduction study and ages above 40 years were considered as independent predictors of disability at 6 months.
The posttraumatic syringomyelia (PTS) is characterized by unexpected insidious progressions of pain and loss of sensorimotor functions which are associated with the development of a fluid-filled syrinx (or multiple separate syrinx cavities) after injury. PTS may develop in patients who have recovered completely from spinal cord injuries as well as in patients without any spinal cord injuries at all. The mean interval between spinal cord injury and diagnosis of PTS ranges from 3 months to 34 years [9].
An injury may trigger syrinx formation by increasing flow in the perivascular space. It appears to be a progression from spinal cord edema (the so-called presyrinx state) to syringomyelia. Once the syringomyelia has developed, the increased intramedullary pressure and fluid movement inside the syrinx may lead to progressive spinal cord damages and neurological symptoms.
In this case, when he was hospitalized in the beginning, his clinical, serological, and electrophysiological features were fully consistent with a diagnosis of GBS. When he was re-hospitalized, we raised awareness for considering the possibilities of other diseases with his given symptomatic changes, including his sensory disturbances in the upper extremities with hyperreflexia, weaknesses in both upper and lower extremities, and urinary dysfunctions. Ultimately, he was diagnosed with syringomyelia coexisting with GBS, which was evident in the cervical spine MRI scans.
To find the cause of syringomyelia, we reviewed his past medical history thoroughly. He had a road traffic accident ten years ago. His truck was severely damaged but he had not had any neurological symptom, abnormal findings in imaging studies and sequelae. It is hard to rule out the possibility of his traffic accident which caused syringomyelia.
It is possible to predict his poor prognosis of GBS by only considering his age and peroneal conduction block in the NCS. If we misdiagnosed his symptomatic changes as a fluctuation or progression of GBS, we might select other inappropriate treatment options such as additional IVIG treatment.
Since a seemingly obvious case of GBS could be complex to diagnose, another underlying causes and the possibility of coexisting additional diseases should always be considered when making a diagnosis to choose better treatment options. After he was diagnosed with syringomyelia, we were able to provide him with appropriate information on the disease and treatments by including a medication to properly control the spasticity.
In conclusion, we report a rare case of symptomatic syringomyelia coexisting with GBS. In such cases, it is necessary to have a careful approach to the diagnosis and keep in mind the possibilities of other coexisting diseases even though there are obvious symptoms of a certain disease.
References
1. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barre syndrome. Ann Neurol. 1990; 27(Suppl):S21–S24. PMID: 2194422.
2. Tse AC, Cheung RT, Ho SL, Chan KH. Guillain-Barre syndrome associated with acute hepatitis E infection. J Clin Neurosci. 2012; 19:607–608. PMID: 22285113.
3. Roser F, Ebner FH, Sixt C, Hagen JM, Tatagiba MS. Defining the line between hydromyelia and syringomyelia: a differentiation is possible based on electrophysiological and magnetic resonance imaging studies. Acta Neurochir (Wien). 2010; 152:213–219. PMID: 19533016.

4. Chen JK, Chen CH, Lee CL, Chen TW, Weng MC, Huang MH. Acute idiopathic syringomyelia: a case report. Kaohsiung J Med Sci. 2004; 20:404–409. PMID: 15473652.

5. Sung WS, Chen YY, Dubey A, Hunn A. Spontaneous regression of syringomyelia: review of the current a etiological theories and implications for surgery. J Clin Neurosci. 2008; 15:1185–1188. PMID: 18710806.
6. Stoodley MA, Jones NR, Yang L, Brown CJ. Mechanisms underlying the formation and enlargement of noncommunicating syringomyelia: experimental studies. Neurosurg Focus. 2000; 8:E2. PMID: 16676925.

7. Batzdorf U. Primary spinal syringomyelia: a personal perspective. Neurosurg Focus. 2000; 8:E7. PMID: 16676930.

8. van Doorn PA, Kuitwaard K, Walgaard C, van Koningsveld R, Ruts L, Jacobs BC. IVIG treatment and prognosis in Guillain-Barre syndrome. J Clin Immunol. 2010; 30(Suppl 1):S74–S78. PMID: 20396937.
9. Klekamp J. Treatment of posttraumatic syringomyelia. J Neurosurg Spine. 2012; 17:199–211. PMID: 22794351.

Fig. 1
This is cervical magnetic resonance image which was taken with symptoms of spasticity attack. (A) C-spine sagittal T2-weighted image show a syrinx between the 5th cervical and the 1st thoracic vertebral segments associated with disc bulging and degeneration with posterior osteophytes (particularly in left C6-7), C5-6 and C6-7. (B) C-spine axial T2-weighted image show chronic myelopathy, C5-T1.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download