Abstract
Neurogenic bladder is a common cause of acute pyelonephritis (APN) in cauda equina syndrome (CES). Perirenal hemorrhage, a rare complication of APN, can be a life-threatening condition. To our knowledge, there is no previous report of perirenal hemorrhage as a complication of APN in CES. A 57-year-old male, diagnosed with CES, due to a L3 burst fracture 3 months earlier, was presented with fever and chills. His diagnosis was APN due to neurogenic bladder. After treatment for APN, he was transferred to the department of rehabilitation medicine for management of his CES. Because of large post-voiding residual urine volumes, he performed self-catheterization after voiding. However, he presented again with fever and chills, and recurrent APN was diagnosed. On the third day of antibiotic treatment, he had acute abdominal pains and hypovolemic shock. Abdominal computed tomography and angiography showed left APN and a perirenal hematoma with left renal capsular artery bleeding. After embolization of the left renal capsular artery, no further active bleeding occurred. Because APN due to neurogenic bladder can lead to critical complications, such as perirenal hemorrhage, the physician should pay attention to the early diagnosis and treatment of urinary tract infection and the management of neurogenic bladder after CES.
Cauda equina syndrome (CES) has various signs and symptoms, such as back pain, sciatic neuralgia, paraparesis, sensory disturbances in the lower extremities, and loss of visceral function. CES is caused by direct or indirect compressive damage, such as herniated intervertebral disc, fracture, and infection in the cauda equina nerve bundle.
Because the cauda equina is a complex of nerve roots located in the distal part of the conus medullaris and includes the S2-S4 roots responsible for the spinal micturition reflex, CES may occur in association with neurogenic bladder [1]. Urinary tract infections (UTIs) are also common in CES due to incomplete voiding of the neurogenic bladder; however, symptoms, such as dysuria, frequency, urgency, abdominal tenderness, and costovertebral angle tenderness are not apparent due to hypoesthesia [2]. Nonetheless, UTI, such as acute pyelonephritis, can cause fatal complications, such as sepsis, renal failure, renal rupture, or perirenal hemorrhage. Spontaneous renal rupture and perirenal hemorrhage following acute pyelonephritis have been reported, albeit rarely [3], and were due to renovascular or renal parenchymal ruptures.
To our knowledge, no reports on perirenal hemorrhage caused by renal capsular artery ruptures have been published so far. Hereby, we present a case of CES that developed in the renal capsular artery rupture and perirenal hemorrhage in which we diagnosed and treated with acute pyelonephritis. We also present a literature review.
A 57-year-old male patient with no specific medical history was admitted to University Hospital for paraparesis and sensory disturbances in both lower extremities, caused by a fall 3 months prior to admission. He was diagnosed with CES, due to a L3 burst facture and spinal canal compression. Thus, posterior lumbar interbody fusion was performed (Fig. 1). After surgery, paraparesis improved progressively and no specific symptoms were reported, except neuropathic pain. The patient was transferred to a rehabilitation hospital for conservative treatment of the neurogenic pain and gait training; no specific complication was found during treatment.
The patient was transferred to our emergency department with fever and general weakness for 7 days. A complete blood count indicated leukocytosis, and Klebsiella pneumoniae was isolated from blood and urine culture tests. By abdominal ultrasonography, edema was observed in the bilateral renal parenchyma with hyperechogenicity, indicating renal failure, caused by acute pyelonephritis. No local lesion in the renal parenchyma, decreased blood perfusion, or extension of the renal pelvis or renal calyx was observed. After diagnosing sepsis, caused by acute pyelonephritis, meropenem was administered intravenously. After antibiotic treatment for 13 days, vital signs were stable and laboratory findings returned to normal. Antibiotics were stopped on the 15th day.
The patient was transferred to the rehabilitation medicine department for comprehensive management of his CES on the 16th day of hospitalization. In the Medical Research Council Manual Muscle Test, muscular weakness was observed in both lower limbs, with hip flexor 4/4 (right/left), knee extensor 3/3, ankle dorsiflexor 3/3, hallucis extensor 3/3, and ankle plantar flexor 2/2 grades. The patient complained of tingling sensations, allodynia, hyperalgesia, and decreased sensations below the third lumbar segment.
Sitting and standing up could be performed independently according to the functional evaluation, but moderate assistance was necessary for balanced-level walking and the Modified Barthel Index was 76 points. The deep tendon reflex was decreased in both lower limbs, though no pathological reflex was observed. Anal sphincter tone and the bulbocavernosus reflex were decreased. An electrodiagnostic study revealed normal sensory nerve conduction in both lower limbs, but the conduction velocity for bilateral peroneal and tibial nerves was slow and the amplitude of the compound muscle action potential was decreased. The H-reflex showed delayed latency in the bilateral tibial nerves. Somatosensory evoked potential stimulating of the bilateral tibial nerves and the bulbocavernosus reflex, stimulating the pudendal nerves, revealed delayed latencies. In needle electromyography, increased insertional activity and abnormal spontaneous activity were observed in muscles innervated from L2 to S2. From these findings, bilateral lumbosacral polyradiculopathy, accompanied by a sacral reflex arc lesion, was diagnosed, and was clinically compatible with CES.
At the time of transfer to our department, the patient was voided with an indwelling catheter. The amount of self-voiding after removing the indwelling catheter was 100-200 mL and the residual urine volume was measured to be about 200-300 mL. Voiding sense and desire were normal, and no incontinence, feeling of residual urine or hesitancy was reported. However, timed intermittent catheterization was used for removal of residual urine after self-voiding.
The patient had sufficient improvements with walking independently on an even surface level through comprehensive rehabilitative managements, including drug therapy (tramadol 150 mg, gabapentin 900 mg) for neuropathic pains, muscle strengthening exercises, functional electrical stimulation for bilateral lower limbs, and gait training. On the 13th day after transfer, the patient was presented with chills and fever (38.5℃). In a complete blood count, leukocytes revealed 26,110/µL, hemoglobin was 9.3 g/dL, and platelets were 168,000/µL. Creactive protein and the erythrocyte sedimentation rate increased to 34.32 mg/L and 120 mm/h, respectively. In serum biochemical tests, the blood urea nitrogen (BUN)/creatinine ratio increased slightly, to 30.9/1.6 mg/dL, and no other specific finding was observed. A routine urinalysis showed a specific gravity of 1.016, pH 6.0, albumin (2+), glucose (3+), ketones (-), hemoglobin (1+), leukocyte (3+), nitrate (-), and red blood cell 1-4/high power field (HPF), and a white blood cell in excess of 5/HPF by high-resolution microscopy. After diagnosis of recurrent acute pyelonephritis, tazobactam, an empirical antibiotic drug, was administered intravenously. However, Klebsiella pneumoniae was isolated from blood and urine cultures, so the antibiotic was changed to meropenem.
After 3 days, the patient suddenly complained of pain and tenderness on the left flank during voiding and a 10×20 cm-sized mass was palpated in the left abdomen. Vital signs at that time showed a blood pressure of 100/60 mmHg, a heart rate of 115 beats/min, a respiratory rate of 20 breaths/min, and an axillary temperature of 36.5℃. In a complete blood count, leukocytes were 28,900/µL, hemoglobin was 7.1 g/dL, and platelets were 158,000/µL, indicating a sharp decrease in hemoglobin; thus, an acute hemorrhage was suspected. In a coagulation profile test, the prothrombin time was 13.7 seconds, the international normalized ratio was 1.21, and the activated partial thromboplastin time was 35.7 seconds, indicating normal findings. In a serum biochemical test, the BUN/creatinine ratio had increased, to 43.1/3.5 mg/dL, but no other specific finding was observed. In contrast-enhanced abdominal computed tomography (CT), pyelonephritis with multifocal low-density lesions was observed in the left kidney and a perirenal hemorrhage of low density was found in the perinephric space. From the arterial to the delayed phase, active extravasation of contrast media was observed near the perinephric space and was thought to be a hemorrhage in a capsular artery outside the renal capsule.
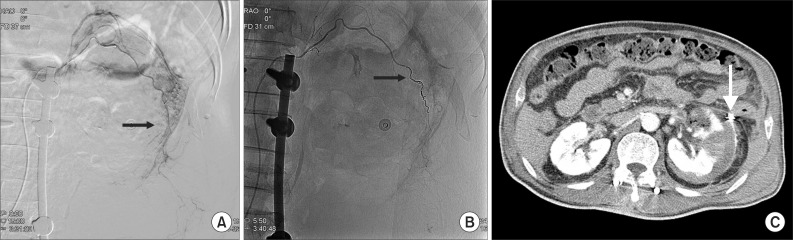
The mass palpated in the left abdomen was found to be a retroperitoneal hematoma with low density in the left iliac fossa (Fig. 2). Systolic blood pressure had decreased by 90 mmHg with declined hemoglobin, thus, emergent transfusion and angiography were performed. No tumor or vascular malformation was found, but coil embolization was carried out due to suspicion of progressive hemorrhage in the left capsular artery (Fig. 3A, B). After transferring the patient to the intensive care unit, conservative treatment for acute renal failure with acute pyelonephritis was performed. No more active bleeding was observed in a follow-up abdominal CT, and the embolized capsular artery was found around the anterior cortex of the left kidney, at the site of the acute pyelonephritis (Fig. 3C).
After coil embolization, the hemorrhage stopped, so, nephrectomy was not being considered. However, surgical hematoma removal was conducted, because of persistent abdominal pains. In voiding cystourethrography, the vesicoureteral reflux was not observed, and no voiding images were obtained because the patient failed to void after filling 450 mL of contrast medium even though he had voiding desire. In an urodynamic study, the patient had strong voiding desire when infused with 500 mL of saline, but failed to void due to detrusor areflexia.
The patient was administered oral medications, including an alpha blocker and a choline agonist, but intermittent self-catheterization was carried out five times per day due to urinary retention in excess of 500 mL. No additional UTIs or other complications occurred, so he was transferred to a rehabilitation hospital.
Spontaneous perirenal hemorrhage was first reported by Wunderlich in 1856, and is a hemorrhage in or around the kidney without a history of trauma. This condition is rare and has non-specific clinical symptoms and various causes, but its mortality rate is high when treatment is late [4]. McDougal et al. [5] reported 78 cases caused by cancer hemorrhage (58%), vascular abnormalities such as polyarteritis (18%), and infection such as perirenal abscess and nephritis (15%).
The typical clinical symptoms are referred to as Lenk triad: sudden abdominal pain, a palpable mass, and hypovolemic shock. Other symptoms, such as nausea, vomiting, and decreased hemoglobin, can also occur [6]. In our case, Lenk triad and decreased hemoglobin were confirmed.
As a diagnostic tool, abdominal CT is most useful for early evaluation and can detect perirenal hemorrhage, renal rupture, and renal tumors. Renal angiography can identify tumors and vascular anomalies which are not detected by CT scanning. Additionally, embolization can be used to control bleeding when needed, so nephrectomy may not be necessary [7].
Acute pyelonephritis is a very rare cause of perirenal hemorrhage and renal inflammation, such as pyelonephritis, can invade the perirenal tissues. A capsular artery derived from the renal artery runs through the perirenal tissues and supplies blood to the perinephric fat. Thus, renal inflammation can spread into perirenal tissues because a capsular artery penetrates the renal capsule and communicates with a subcapsular artery inside the kidney [8].
In our case, neither the renovascular nor the renal cortical rupture was found in a patient with acute pyelonephritis. However, hemorrhage occurred in a capsular artery around the original inflammation. Rupture of a capsular artery might have been caused by erosions in the artery due to the spread of pyelonephritis through communication between a capsular and a subcapsular artery. Regarding the pathophysiology of perirenal hemorrhage, increased pressure in a subcapsular artery, sudden renal congestion, and hydronephrosis have all been mentioned previously [9]. Hemorrhage accompanying renal infection caused by erosions in a vessel has been reported [3].
Generally, the aim in treating neurogenic bladder that occurs after CES is to choose a voiding method taking into account the patient's functional level, and prevent complications, including infections, hydronephrosis, renal failures, urolithiasis, or autonomic dysreflexia [10]. If UTI combined with CES develops, secondary complications can be life-threatening, such as perirenal hemorrhage, should be considered. Life-threatening complications can also be prevented by selecting an appropriate voiding method through proper evaluation of neurogenic bladder, preventing UTI, and early treatment. We treated CES with non-traumatic perirenal hemorrhage and experienced hemorrhage in a capsular artery, which to our knowledge, has not yet been reported.
References
1. Gitelman A, Hishmeh S, Morelli BN, Joseph SA Jr, Casden A, Kuflik P, et al. Cauda equina syndrome: a comprehensive review. Am J Orthop (Belle Mead NJ). 2008; 37:556–562. PMID: 19104682.
2. Shin JC, Park CI, Rha DW, Chon J, Kim JE, Jeon SC, et al. The diagnosis of upper urinary tract infection using abdominal computerized tomography in spinal cord injured patients. J Korean Acad Rehabil Med. 2004; 28:140–145.
3. Song HJ, Choi MY, Kim MY, Lee YS, Kim SJ, Choi GB, et al. A case of spontaneous rupture of the kidney with acute pyelonephritis. Korean J Nephrol. 2003; 22:757–762.
4. Chang TH, Wu WJ, Hsiao HL, Yeh HC, Huang CH, Lee YC. Spontaneous perirenal hematoma: a case report. Kaohsiung J Med Sci. 2005; 21:578–581. PMID: 16670051.

5. McDougal WS, Kursh ED, Persky L. Spontaneous rupture of the kidney with perirenal hematoma. J Urol. 1975; 114:181–184. PMID: 1159905.

6. Albi G, del Campo L, Tagarro D. Wunderlich's syndrome: causes, diagnosis and radiological management. Clin Radiol. 2002; 57:840–845. PMID: 12384111.
7. Yoon JC, Kim W, Cho GC, Hong JS, Lee MW, Jang SE, et al. Idiopathic spontaneous renal rupture. J Korean Soc Emerg Med. 2001; 12:523–527.
8. Aizenstein RI, Owens C, Sabnis S, Wilbur AC, Hibbeln JF, O'Neil HK. The perinephric space and renal fascia: review of normal anatomy, pathology, and pathways of disease spread. Crit Rev Diagn Imaging. 1997; 38:325–367. PMID: 9376088.
9. Polkey HJ, Vynalek WJ. Spontaneous nontraumatic perirenal and renal hematomas: an experimental and clinical study. Arch Surg. 1933; 26:196–218.
10. Bryce TN, Ragnarsson KT, Stein AB. Spinal cord injury. In : Braddom RL, editor. Physical medicine and rehabilitation. 3rd ed. Philadephia: Sauders;2007. p. 1328–1329.
Fig. 1
Initial T2-weighted magnetic resonance imaging scans show (A) burst fracture at L3 vertebral body and (B) retro-pulsed bony fragments into spinal canal. (C) Postoperation X-ray demonstrates posterior fixation state in L1-4.
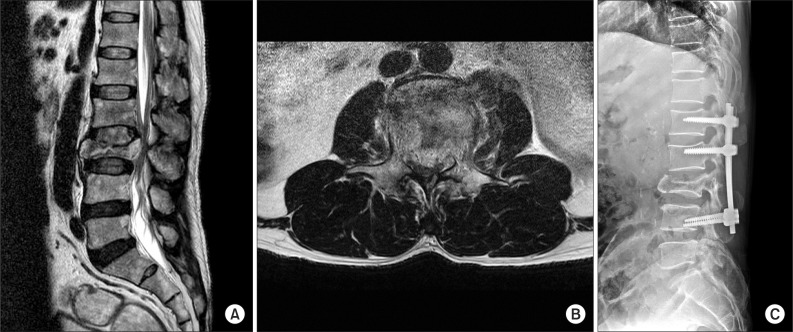
Fig. 2
Contrast enhanced abdominal computerized tomography shows (A) multiple hypoattenuation of left kidney parenchyme (asterisk) and hypoattenuated fluid collection with density of blood in the perinephric space (arrow), (B) active extravasation of contrast media at the left renal capsular artery (arrow), and (C) hypoattenuated fluid collection with contrast leakage in lower left quadrant pelvic cavity (arrow).
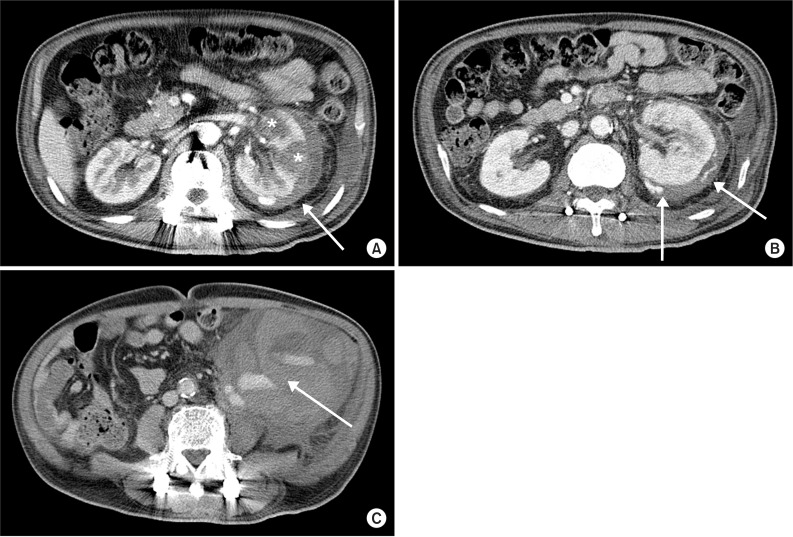
Fig. 3
Selective left renal angiography shows suspicious contrast leakage of left renal capsular artery (A, arrow) and no more contrast leakage after renal capsular artery embolization with microcoils (B, arrow). Follow-up abdominal computerized tomography shows no visualization of active bleeding and embolization state with microcoils in left renal capsular artery (C, arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download