INTRODUCTION
The plantar fascia originates on the inferior side of the calcaneus and is attached to the plantar plate of the metatarsophalangeal joint and the base of the proximal phalanx of the toe. It is important in maintaining the medial longitudinal arch and in absorbing shocks, and is also involved in the windlass mechanism during walking [
1,
2]. When excessive loads are repeatedly applied to the plantar fascia, acute or chronic inflammatory changes occur in the calcaneus insertion, leading to the development of plantar fasciitis [
1,
3-
6]. Plantar fasciitis is the most common cause of heel pain. Cole et al. [
7] reported that plantar fasciitis is a common condition that occurs in about 10% of the American population.
Generally, pain is aggravated when a patient with plantar fasciitis takes their first steps in the morning but gradually improves with activity. Another characteristic of the condition is the experience of tenderness when pressure is applied to the medial calcaneus on which the plantar fascia originates. Also, when the toes are dorsiflexed, the plantar fascia contracts, and pain increases [
3,
8,
9]. While the precise cause of plantar fasciitis is unknown, it is associated with obesity, middle age, flat foot, and pes cavus [
7].
Treatment methods for plantar fasciitis are diverse, including medication, physical therapy such as stretching exercises, non-surgical treatments such as insole, night splint, and local steroid injection, and surgical treatments such as plantar fascia release. However, the results of the treatment were not consistent [
7,
10].
Recently, many researchers have demonstrated the effects of extracorporeal shock wave therapy (ESWT) on chronic plantar fasciitis that has previously been resistant to conservative treatment. Their studies have shown that ultrasonography not only alleviates subjective pain but also changes the thickness of the plantar fascia [
11-
15]. While a number of papers have reported the mechanism and effects of ESWT on plantar fasciitis, no treatment protocol for ESWT has been established. Particularly, much controversy exists regarding the proper amount of energy to be applied to the affected tissue.
Our aim in this study was therefore to investigate the dose-related effect of shock wave therapy at different total energy influx by adjusting the times of sessions and energy flux density (EFD) in patients with chronic plantar fasciitis.
Go to :

DISCUSSION
As a fiber tissue that originates on the medial calcaneus, the plantar fascia is attached to the forefoot along with various other tissues. Segmented into the medial, central, and lateral aponeurosis, the central aponeurosis is the origin of most problems. The cells in the plantar fascia consist of collagen and elastic fibers, and changes in the arrangement of the elastic fibers due to excessive loads can cause a stiffening of the fascia.
The conservative treatment methods for plantar fasciitis include the discontinuance of the exercises or activities that cause pain, stretching of the Achilles tendon and plantar fascia, heel pad, insole, night splint, anti-inflammatory drugs, and steroid injection. The effects of each treatment are presented with varying results according to different studies, and the most effective treatment method has not been clearly identified [
17,
18]. Furthermore, the success rates of these conservative treatments are reported to range from 44% to 90%; some researchers have also noted that the implementation of conservative treatments for plantar fasciitis is difficult [
18-
22]. ESWT is largely used in the treatment of plantar fasciitis given that the therapy is noninvasive, enables an immediate return to ordinary life, and its effects are similar to surgical interventions [
23].
The mechanism of ESWT causes excessive excitement of the axon, affecting topical pain factors, thereby generating a reflexive analgesic effect and reduces pain by destroying unmyelinated sensory fibers. Moreover, it is known to improve the symptoms of the condition by an inflammatory response through the secretion of growth factors or nitrous oxide; it also repairs damaged tissues by encouraging angiogenesis [
24-
26].
However, not all the relevant studies have reported positive results regarding the effects of ESWT on plantar fasciitis. Indeed, some researchers have reported that ESWT was not effective when compared to the control group [
27,
28]. The reasons for such varied ESWT results include applicator position [
29], the use of local anesthesia [
30], and most importantly, the corresponding diverse intensity levels, defined as the energy flow through an area with perpendicular orientation to the wave propagation [
31,
32].
ESWT can be divided into different energy influx levels. Some previous studies [
32,
33] have divided the ESWT treatment intensity into three levels: 1) low intensity (EFD<0.08 mJ/mm
2); 2) medium intensity (EFD, 0.08-0.28 mJ/mm
2); and 3) high intensity (EFD>0.28 mJ/mm
2). While the intensity or delivered energy is considered by some researchers to be a key factor for successful treatment [
14,
31], in current literature reviews, debate continues over the appropriate energy intensity and the total delivered energy that should be applied to the tissue.
While some studies that advocate the use of high-intensity energy report that it enables a single treatment and the treatment effects are superior [
14,
23], an increased energy influx within a short period of time results in a corresponding increase in pain, local swelling, and tenderness. Therefore, higher intensity treatments usually require local anesthesia, which is known to reduce the efficacy of the treatment [
30]. Furthermore, some animal tests report that an influx of energy of over 0.60 mJ/mm
2 can cause permanent damage on the tendon [
34]. On the other hand, low-intensity energy is safer but has the disadvantage of lower treatment effects [
25].
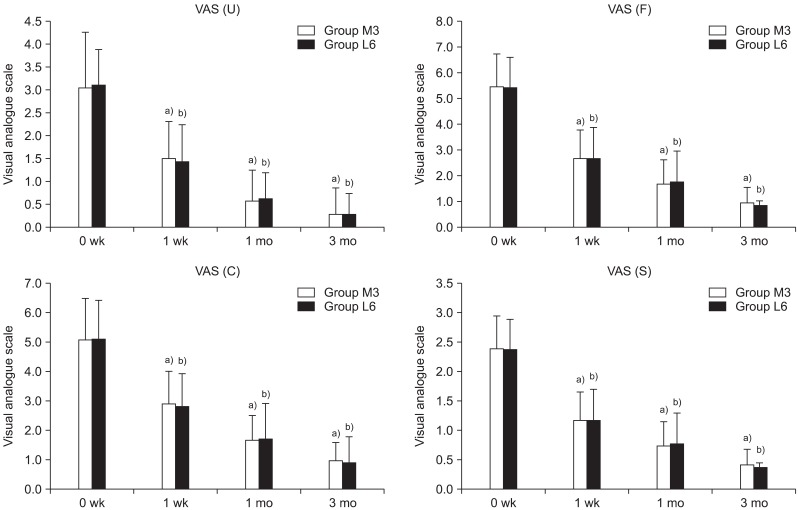
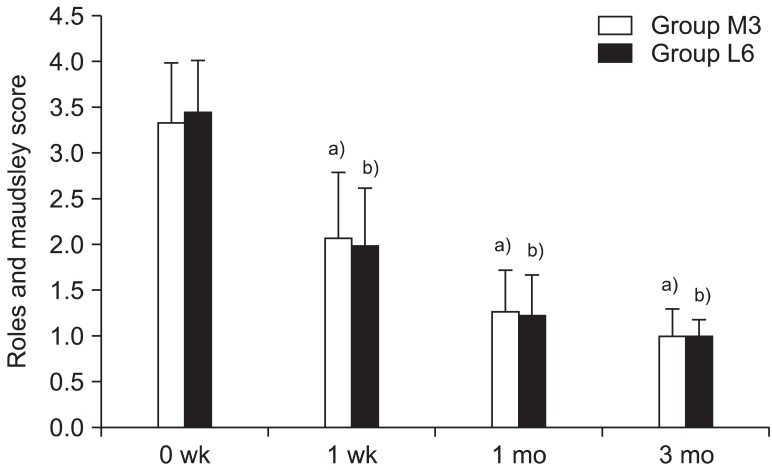
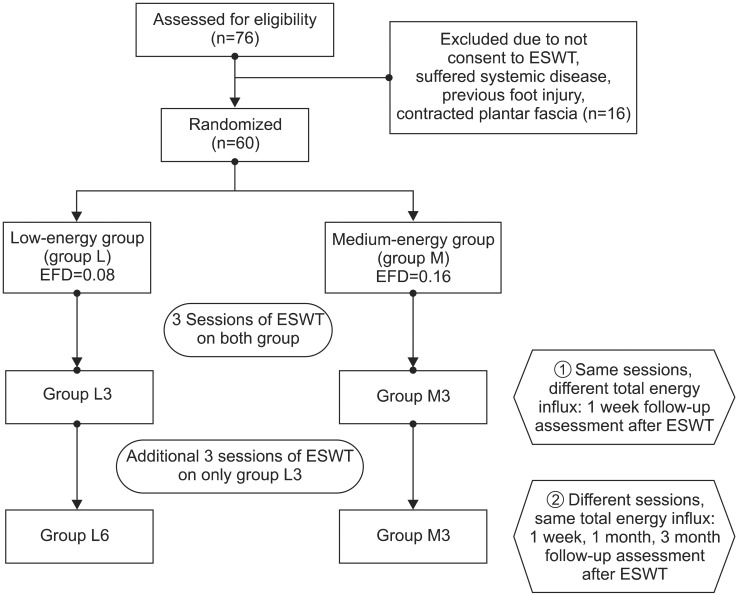
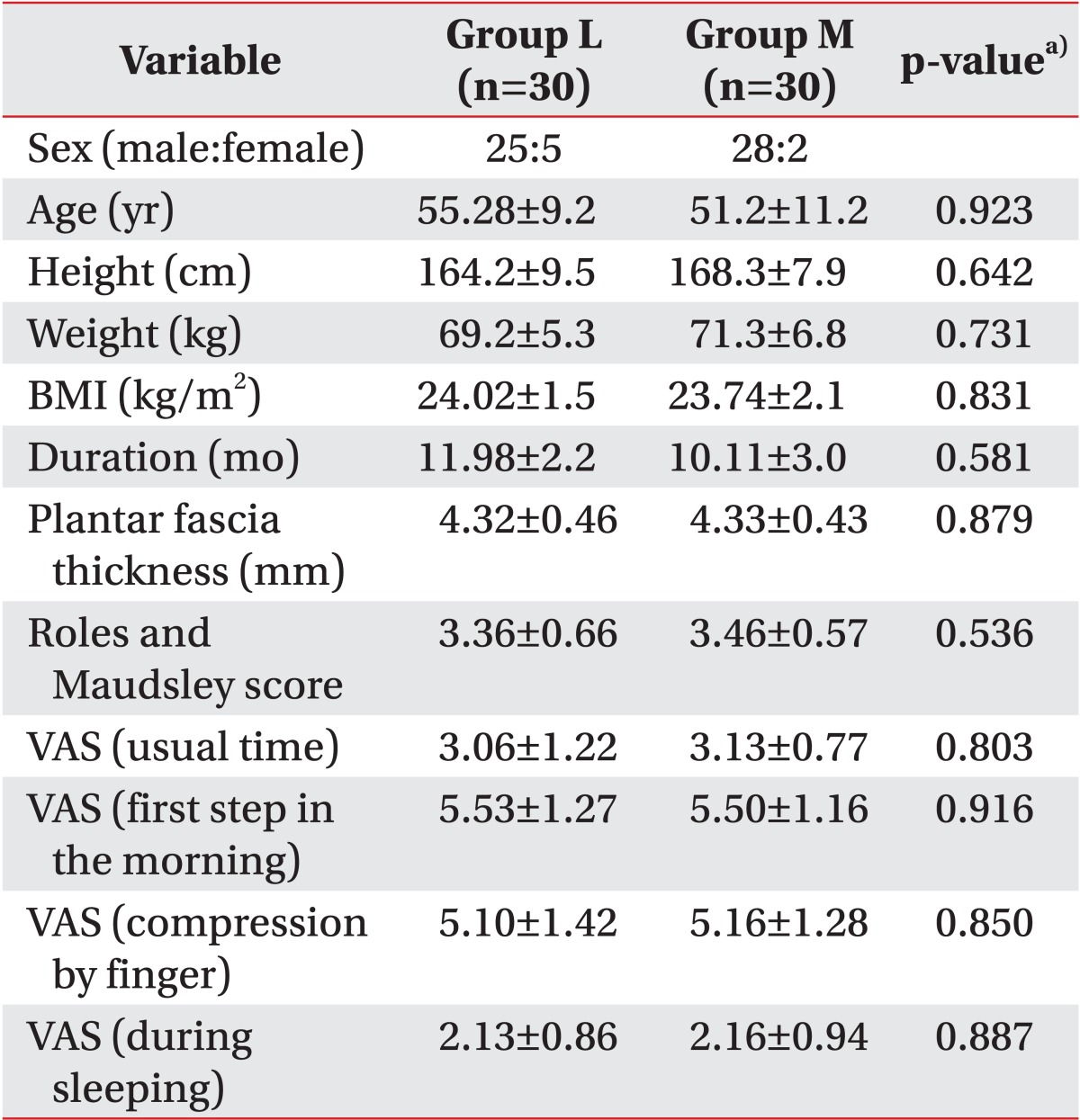
In this study, the treatment effects in the two groups were compared; groups L and M had low energy of 0.08 mJ/mm
2 applied for six sessions and medium energy of 0.16 mJ/mm
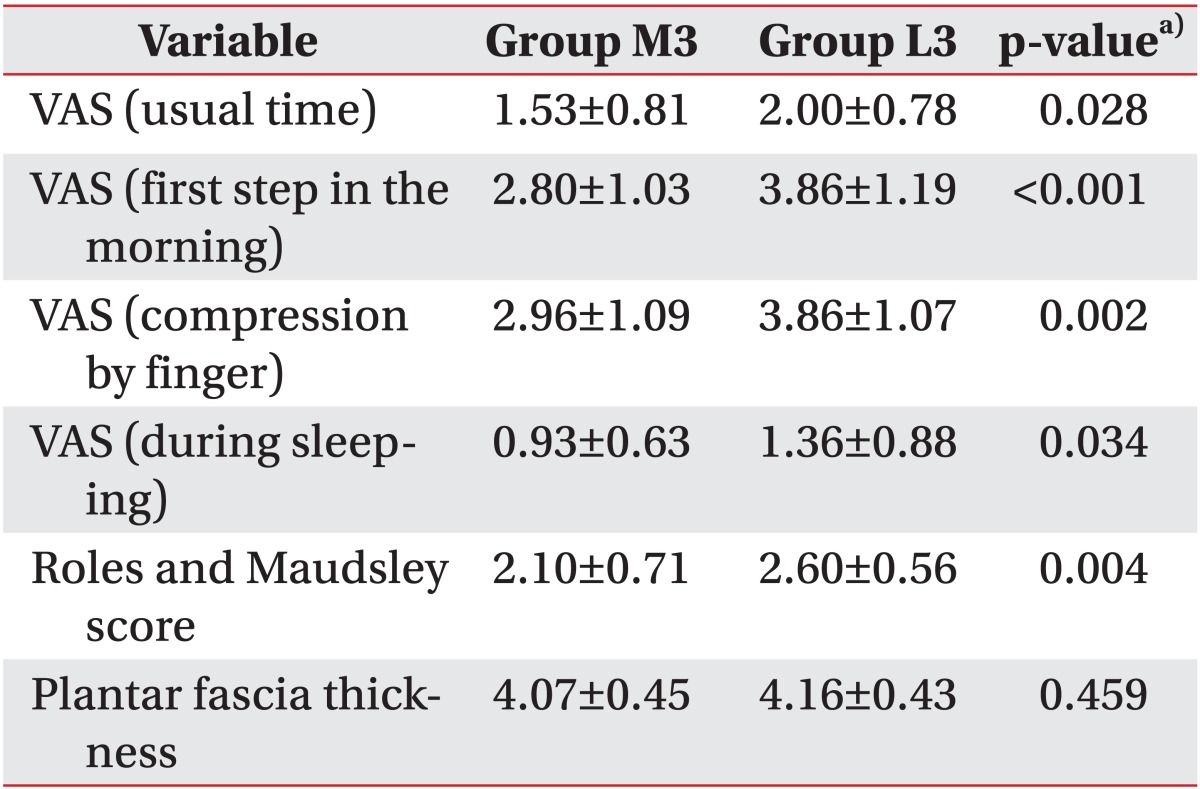
2 applied for three sessions, respectively. The medium-energy group showed statistically significant pain reduction and function scores improvement compared to the low-energy group when the same sessions were applied. This concurs with the results of previous studies, which demonstrated that higher-intensity energy destroys more unmyelinated sensory nerve fibers and thereby has a greater pain-reducing effect [
35,
36].
No standardized guidelines are available for the number of sessions needed when using ESWT in soft tissue condition [
24]. However, numerous clinical studies have reported using two or three sessions for treating chronic tendinopathy, though this may reflect the clinical experience of the researchers. Takahashi et al. [
35] reported the cumulative effects of repeated shock waves with single and double applications on nerve fibers and demonstrated that both groups showed nearly complete degeneration of epidermal nerve fibers until the fourth week of treatment. However, by the end of the sixth week of treatment, a reinnervation of the epidermis was detected in the single-treatment group, whereas reinnervation occurred more slowly in the repeat-treatment group. Therefore, they suggest multiple applications of ESWT provide longer-lasting nociceptive effects. Notwithstanding, their study differed from the current study since the researchers merely compared a single treatment with double applications while applying the same low energy, and investigated the number of degenerated nerve fibers instead of clinical indices. Additionally, no reports have been published regarding the time point of nerve reinnervation after multiple applications of three and six sessions, as given in the present paper.
In this study, an identical amount of total influx energy was applied to the two groups in a differentiated treatment schedule comprising of three and six sessions for groups M and L, respectively. In this case, both groups exhibited analgesic effects until three months after the end of the treatment. This demonstrates a longer-analgesic effect representing the cumulative effects of ESWT. In this study, the low-energy group with six sessions and the medium-energy group with three sessions, which each received the same total amount of energy influx, showed no differences in the scores of pain, function, and thickness of the plantar fascia. This may suggest that the effects of ESWT in plantar fasciitis are related not only to EFD but also to the times of sessions and total energy influx.
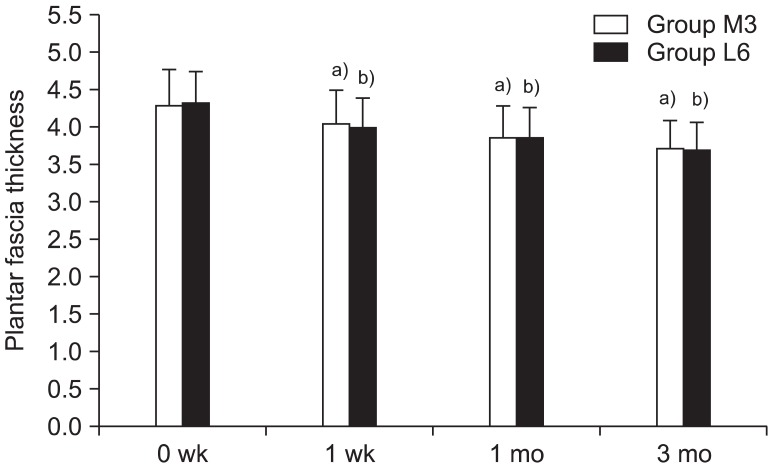
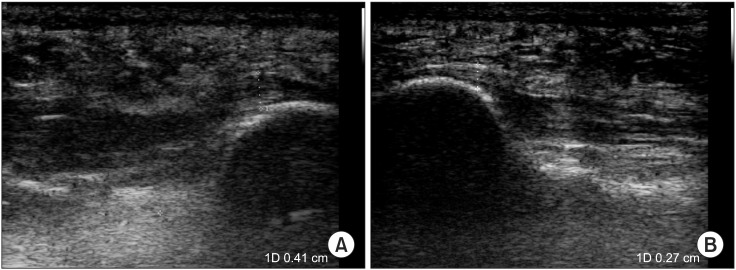
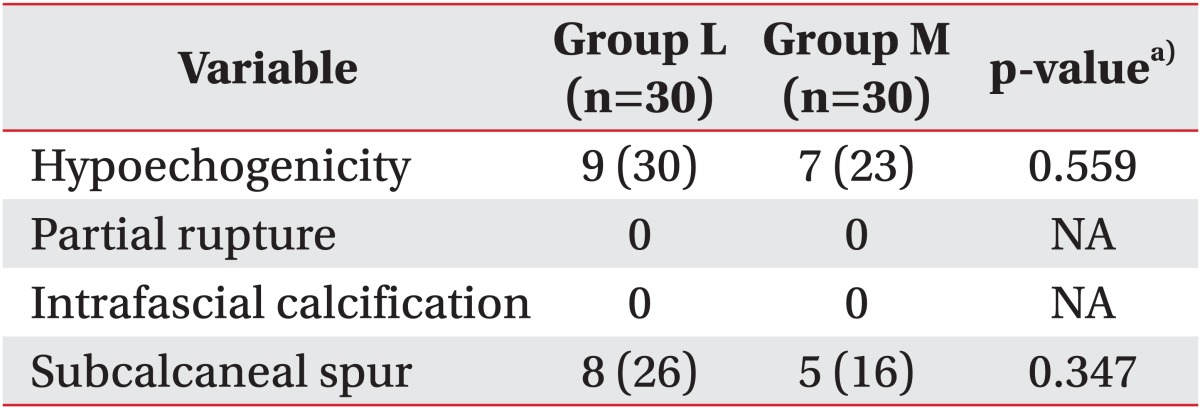
When plantar fasciitis is prolonged, it is known to reduce blood circulation around the plantar fascia, and subsequently cause a thickening of the plantar fascia. Previous studies [
15,
37] noted that ESWT revitalizes tissues by increasing angiogenesis and nitric oxide mediated anti-inflammatory effect, which thereby decreases the thickening of the plantar fascia by stabilizing inflammation. In this study, both groups exhibited a statistically significant decline in the thickness of the plantar fascia over time, which demonstrates a similar result to that of previous studies [
13-
15]. However, no differences in the thickness of the plantar fascia were detected between the medium-energy group with three sessions and the low-energy group with three sessions. Furthermore, when the two groups were compared one month and three months after the additional 3 sessions of ESWT were conducted on the low-energy group, no statistically significant differences were observed. Hammer et al. [
15] reported the reduction of the plantar fascia was stable between 6 to 24 weeks after treatment. The result of our study, which shows no significant difference in plantar fascia thickness according to energy level, is most likely because the reduction of plantar fascia was not fully completed within the follow-up period. Calcagni et al. [
38] compared the microvascular responses between two groups by applying different total energy influx, which comprised of 500 and 1,000 shocks at each session with low energy of 0.08 mJ/mm
2. Both groups showed increased functional capillary density (FCD) and up-regulation of the proliferating cell nuclear body (PCNA). This tissue reaction started immediately after ESWT and slowly declined over a period of 3 days and 3 days after ESWT, the level of FCD and PCNA show no significant difference in both groups. The findings of their study concluded that there were no differences between the two groups when comparing the degrees of angiogenesis resulting from differentiated total energy influx. This supports the results of the current study in that no differences in the thickness of the plantar fascia resulted from differentiated total energy influx.
Contrary to the concerns that higher-intensity energy would be more likely to cause side effects, the medium-energy group in the present study did not show notable side effects that would cause treatment to be halted. This appears to be in accordance with the findings of an animal study in which it has been shown that tissue changes resulting from an energy of 0.28 mJ/mm
2 or lower were reversible and could be recovered completely within four weeks [
34].
In this study, ESWT with medium energy generated an effect identical to that of ESWT with low energy, but in half of the sessions required for same effect. This indicates that medium energy can be used as an energy level that increases patient-treatment compliance and is safe to apply. In addition, given the results of previous studies [
23,
39] in which medium-energy and high-energy groups exhibited no difference in terms of treatment effects, our findings suggest effective energy levels for pain reduction without side effects.
The limitations of this study are that no control group was employed to exclude placebo effects, no comparison of the treatment effects was made with a high-energy group, and the number of subjects was relatively small, with 30 patients in each group. Future studies will thus be necessary.
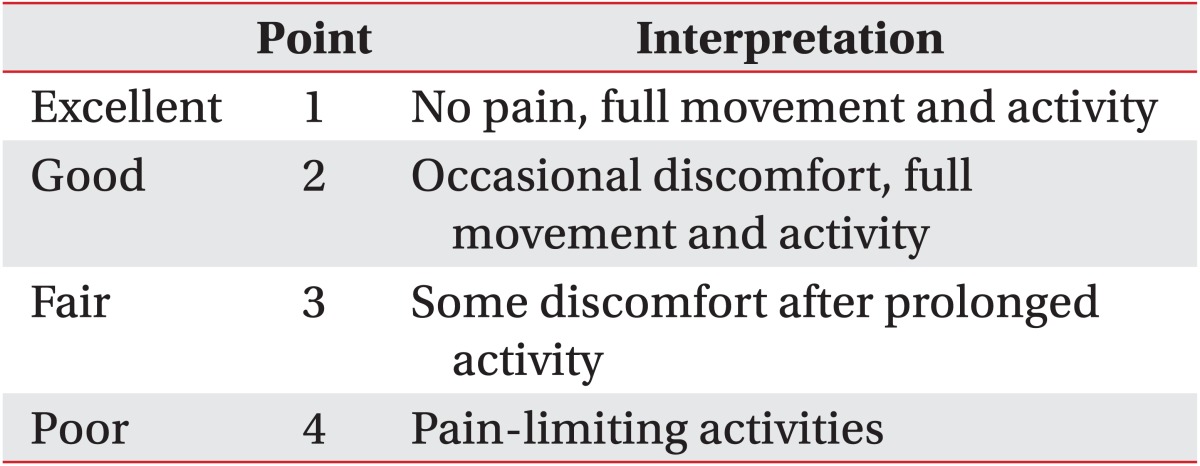
In conclusion, we aimed to evaluate the dose-related effect of ESWT by adjusting the times of sessions and EFD. In applying ESWT to the plantar fasciitis, the adoption of medium-energy level (0.16 mJ/mm2) was more efficient in terms of relieving pain and restoring functional activity than low-energy level (0.08 mJ/mm2) in the same session and showed no adverse effect. However, when different times of sessions were applied for the same total energy influx, different therapeutic effects in the different EFD groups no longer occurred. Therapeutic effect might disclose a dose-related relationship; therefore, EFD and times of sessions are considerable factors when treating with ESWT.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download