INTRODUCTION
Burkitt lymphoma is one of the B-cell lymphomas. Children are more susceptible to it than adults. Clinically, it is classified into three types, i.e., an endemic variant that is related to the Epstein-Barr virus and is prevalent in Africa, a sporadic variant prevalent in regions other than Africa, and an immunodeficiency-associated variant prevalent amongst patients whose immune systems have been impaired due to human immunodeficiency virus (HIV) infection or immunosuppressants used after organ transplants. In particular, the sporadic variant is known to occur in the ileum and the appendix. If Burkitt lymphoma is not treated properly, can lead to death. However, intensive chemotherapy is associated with a very high rate of complete remission.
1
Cytarabine (ara-C) is used for central nervous system prophylaxis during chemotherapy for Burkitt lymphoma, but the patient may develop neurotoxicities (an incidence rate of 5 to 50%) such as ataxia caused by cerebellar injury, ataxic dysarthria, oculomotor dysfunction, confusion, somnolence and lethargy.
2-
5 Such adverse reactions are mild or temporary, but in rare cases they may be severe or long lasting.
2,
4 This paper is a report of a patient with rectal Burkitt lymphoma who showed ataxia caused by cerebellar injury after chemotherapy including cytarabine administration.
Go to :

CASE REPORT
A 55-year-old man, who was a hepatitis B carrier and who took antihypertensive drugs for hypertension, complained of persistent constipation. A colonoscopy was performed in February of 2010. A tumor was observed in the rectum. A palliative colostomy was performed on March 5, 2010, and the patient was diagnosed with Burkitt lymphoma on pathologic examination. With the pre-phase COP regimen (cyclophosphamide, vincristine and prednisolone), chemotherapy was given from March 15, 2010. It was continued with the R-hyper-CVAD regimen (rituximab, cyclophosphamide, mensa, vincristine, adriamycin and dexamethasone) from March 18, 2010. Notable adverse effects were not reported till then. Additional chemotherapy with the R-HD-MTX/Ara-c regimen (rituximab, methotrexate and cytarabine) was given from April 9, 2010. Involuntary movements and ataxia were observed 4 and 7 days, respectively, after the therapy. After chemotherapy with the CALGB course-IIA regimen (cyclophosphamide, 6-mercaptopurine, cytarabine, vincristine and L-asparaginase) was given on May 4, 2010, ataxia worsened and ataxic dysarthria, dysphonation and dysphagia were newly developed. Chemotherapy was ceased due to the neurological adverse effects. Chemotherapy with the R-hyper-CAVD regimen was re-given on July 9 and August 12, and cytarabine, methotrexate and hydrocortisone were intrathecally administered on August 14 for the purpose of central nervous system prophylaxis. Although he was diagnosed as being in complete remission from Burkitt lymphoma, he showed drowsiness and neutropenic fever. Progress was observed with conservative treatment. On brain magnetic resonance imaging (MRI) in April 2010, when neurological adverse effects occurred, notable findings were not observed.
The patent was transferred to the department of rehabilitation medicine at our hospital, where he underwent physical therapy, occupational therapy and pharmacotherapy. His MMSE score was 29 (maximum score=30) as the patient was not able to complete drawing pentagons due to tremor of his arms. On the cerebellar function test, intentional tremors were observed. On the finger-to-nose test, dysmetria was observed on both upper extremities. Also, clumsiness was observed on the rapid alternating movement test and the heel-to-shin test. Cranial nerve examination did not show abnormal findings. On the manual muscle test, both arms and legs were classified as grade-IV in accordance with the Medical Research Council Grade. On the joint range of motion test, contracture was not observed. Spasticity and upper motor neuron signs were not observed. There was no abnormal finding on sensory examinations. Bladder and bowel function were normal. Ataxia and intentional tremors were so severe that the patient had difficulty on almost all activities of daily living. His score on the International Cooperative Ataxia Rating Scale (ICARS) was 91 (
Table 1); on the Functional Independence Measure (FIM) it was 61 (
Table 2). Functionally, the patient could come to a sitting position from laying on a bed (holding a bar), sit alone, and stand up with holding a bar; but he was not able to stand up by himself. Also, he was fed through a nasogatric tube due to dysphagia. Electromyography was done on October 4, 2010 to rule out polyneuropathy caused by anticancer drugs, but abnormal findings were not observed. On a brain MRI and a spinal MRI performed on October 28, 2010, abnormal findings were not observed (
Fig. 1). However, a decrease in 18 F-fluodeoxyglucose uptake was observed in frontal lobes, parietal lobes, temporal lobes and the cerebellum on brain positron emission tomography (PET) performed on October 29 (
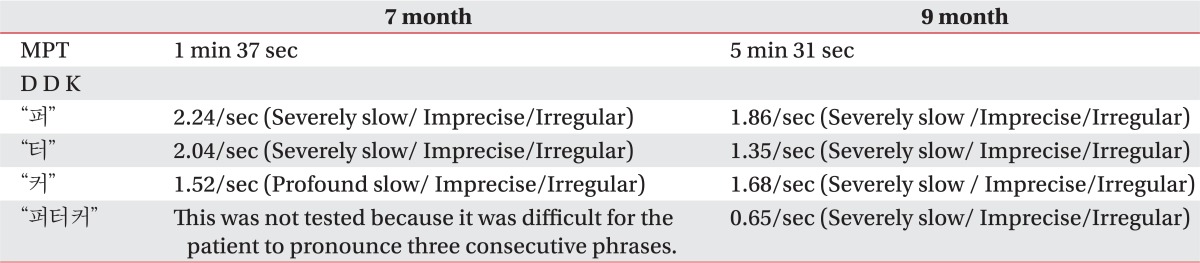
Fig. 2). On a video fluoroscopic swallowing study performed at the end of September, 2010, a delayed swallowing reflex, decreased laryngeal elevation and the penetration of semisolid diet were observed, but other notable findings were not observed. Hence, diets were gradually changed from a level-1 dysphagia diet to a general diet. On the Korean-Western Aphasia Battery (K-WAB), the aphasia quotient was the 81st percentile, and it did not fall into aphasia. On the speech evaluation just 2 or 3 syllables could be articulated during an inspiration, and, generally, consonants and vowels became inarticulate. In the case of speech intelligibility, his speech was difficult to understand without contextual clues. Also, the patient experienced severely harsh voices. Moreover, excessive involuntary movements made it difficult to maintain respiration during vocalization (
Table 3).
 | Fig. 1Brain MRI T2 Flair (2010. 10.28). There were no abnormal findings such as cortical atrophy or cerebromalatic changes in the cerebellum, but right sinusitis was observed. 
|
 | Fig. 2Brain PET CT (2010.10.29). Diffuse hypometabolism was observed in the bilateral frontal, parietal, temporal cortex areas and cerebellum. 
|
Table 1
International Cooperative Ataxia Rating Scale
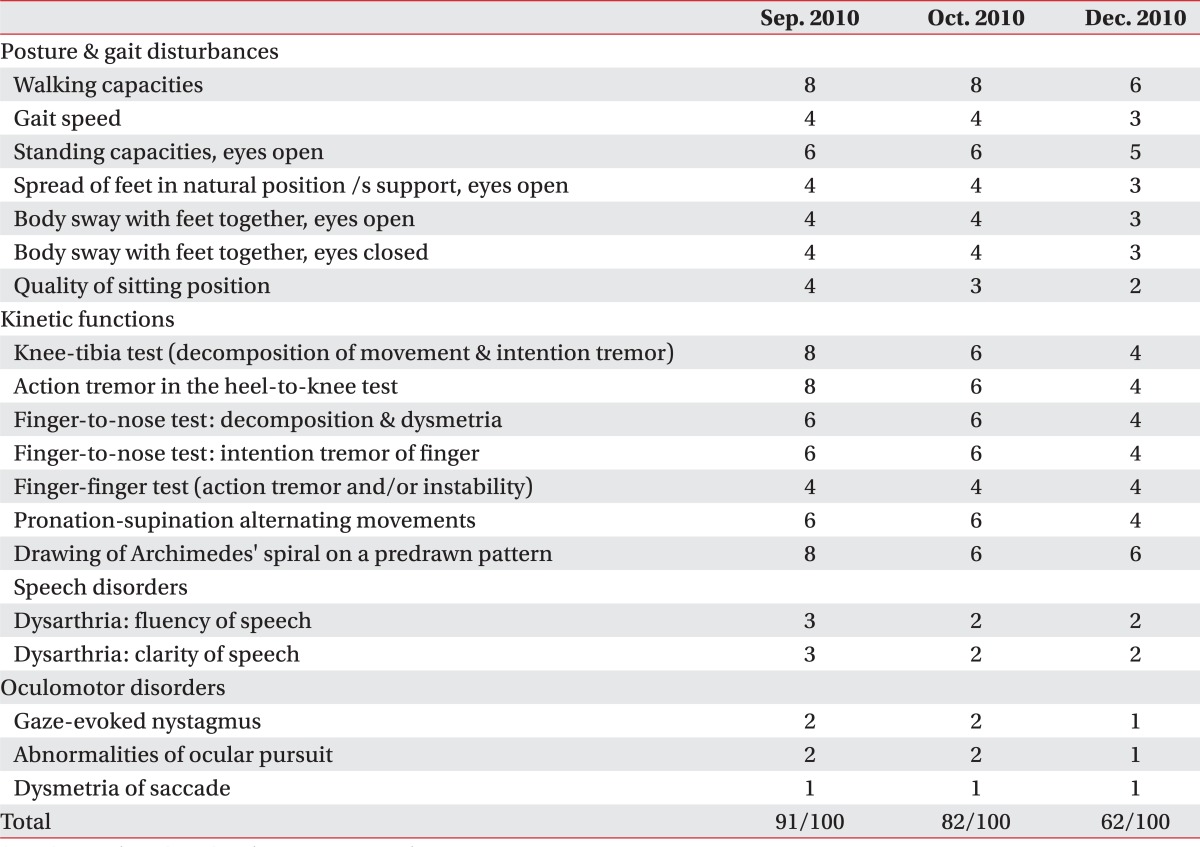

Table 2
Functional Independence Measurement Score
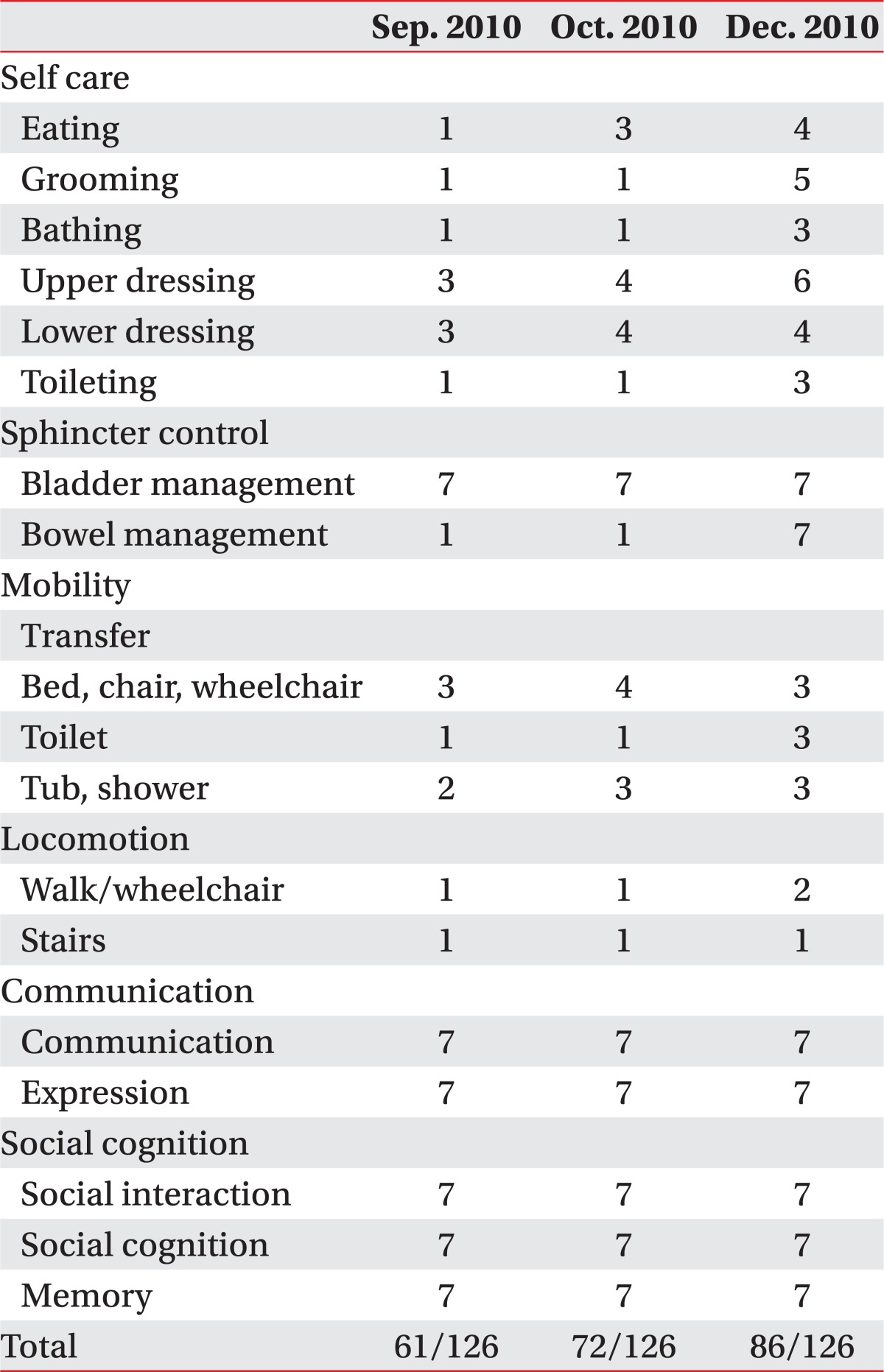

Table 3
Speech Test after Symptom Developed

The patient was hospitalized in the department of rehabilitation medicine at our hospital and underwent physiotherapy (30 minutes at a time, 2 times a day, 5 times a week), occupational therapy (30 minutes at a time, 2 times a day, 5 times a week) and speech therapies (40 minutes at a time, 2 to 3 times a week) in September, October and December of 2010, respectively. His ataxia and intentional tremors improved and he was able to stand alone and walk with minimal assistance. The ICARS score was lowered from 82 in September of 2010 to 62 in October of the same year (
Table 1), and the FIM score improved from 61 in September of 2010 to 89 in December of the same year (
Table 2). On speech evaluation, articulation and vocalization were improved as compared to the results seen in September and December of 2010 (
Table 3). On a PET scan done on February 25, 2011, significant changes were not observed (
Fig. 3).
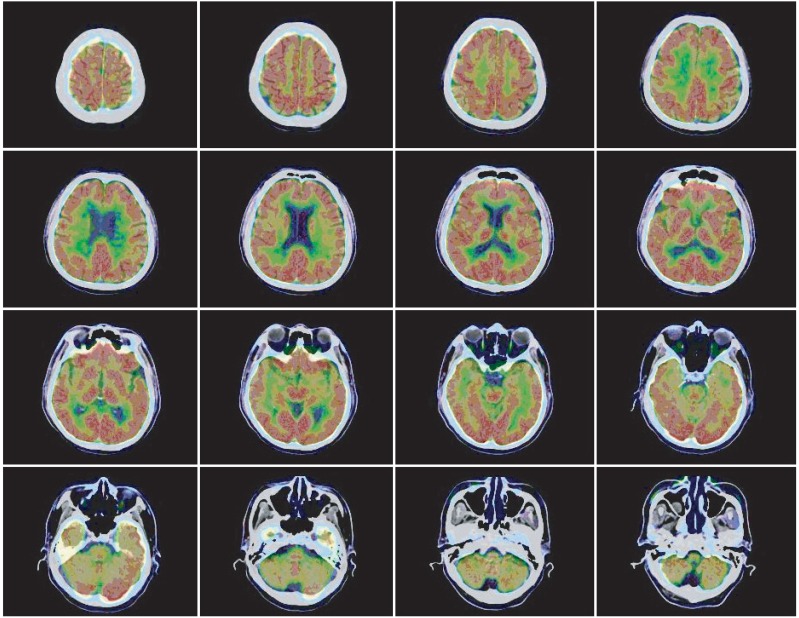 | Fig. 3Brain PET CT (2011.2.25). Compared with a previous study, no interval change was observed (2010.10.29). 
|
Go to :

DISCUSSION
Central nerve system injuries may occur in patients who receive a high dose cytarabine therapy after being diagnosed with lymphoma. In particular, cerebellar injuries are common, which correlate with the results of previous autopsies that Purkinje cells are degenerated or decreased in the cerebellar hemisphere or vermis, as well as with results from brain MRIs showing the diffuse cerebellar atrophy.
2,
6 The cerebellar injury may cause ataxia, ataxic dysarthria, dysphonia, oculomotor dysfunction, confusion, somnolence and lethargy.
4,
5 Ordinarily, such symptoms appear 2 to 4 days after the administration of cytarabine, although the severity varies. They disappear within several weeks in most cases, but may last several months or become permanent in rare cases.
2,
4 The dose of cytarabine, patients aged 60 and older, a history of neurologic dysfunction, hepatic dysfunction and renal insufficiency have been reported to be risk factors.
3,
7
In the case of this patient, ataxia, ataxic dysarthria, dysphonia and dysphagia occurred after the administration of cytarabine, which implied dysfunction of the entire brain, especially the cerebellum. Unlike previous cases where cerebellar atrophy was observed on a brain MRI, abnormal findings were not observed on brain and spinal MRIs performed 6 months after symptoms developed.
6 The previous cases were relevant to patients aged 2 and 6 whose neural development was on going.
6 High-dose cytarabine therapy might inhibit neural development and as a result, cause cerebellar atrophy.
The patient's symptoms gradually improved during physical therapy, occupational therapy and speech therapy, but lasted over 10 months. It is different from the previous cases that the side effects of high dose cytarabine therapy was improved within several weeks. Our patient was a hepatitis B carrier. As noted above, hepatic dysfunction is a risk factor for the adverse effects of cytarabine on the nervous system.
7,
8 Thus, in the case of this patient, the nervous system was more likely to be injured despite the same dose.
This paper is a report of a patient who showed central nervous system adverse effects including ataxia after the administration of cytarabine for rectal Burkitt lymphoma. Abnormal findings were not observed on brain and spinal MRIs, but a brain PET showed diffuse hypometabolism, especially in the cerebellum. The patient's symptoms were improved after rehabilitation therapy.
Go to :


