Abstract
Rotavirus encephalopathy (RE) is a benign afebrile seizure associated with acute gastroenteritis caused by rotavirus infection. We investigated the diffusion tensor tractography (DTT) findings of a patient with RE. The patient was a 30-month-old female that had experienced a brief, generalized convulsive seizure. On the day of admission, the patient had vomiting and experienced watery diarrhea. Her stool was positive for rotavirus antigen. At onset, the patient displayed a drowsy and delirious mental status; later, a splenial lesion of the corpus callosum was found on MRI. One week later, the patient's condition improved and the splenial lesion had disappeared by conventional MRI. Initial DTI showed decreased fractional anisotropy (FA) values of fornix, as well as of the corpus callosum. A follow-up DTT showed a restored interrupted right fonical crus and increased FA values of corpus callosum and fornix. These results highlight the implications of the probability of not only a corpus callosum injury, but a fornix injury as well, in this patient with RE.
Rotavirus encephalopathy (RE) is an acute gastroenteritis (AGE)-induced encephalopathy. Human rotaviruses are the most common agents of sporadic episodes of gastroenteritis in infants and young children on a worldwide basis. AGE-induced encephalopathy, including RE, has been reported to be associated with repeated convulsions during simultaneous episodes of AGE. Although the majority of the patients with AGE-induced encephalopathy recover completely, some patients sustain severe sequelae such as mental retardation, subsequent afebrile seizures, and even death.1 Recent studies of patients with mild encephalitis or encephalopathy reported a reversible lesion in the splenium of the corpus callosum (SCC) on conventional magnetic resonance imaging (MRI);2 this has led to the recognition of a new clinical-radiological syndrome called mild encephalitis/encephalopathy with reversible splenial lesion (MERS).3,4 Drowsy or delirious behavior seems to be one of the main clinical features in patients with MERS.3,4 However, these studies were mainly conducted using conventional brain MRI, and did not reveal the microstructural status of white matter pathology. The recent development of diffusion tensor imaging (DTI) allows the evaluation of the integrity and orientation of neural tracts at the subcortical level. Diffusion tensor tractography (DTT), a 3-dimensional visualized version of DTI, is useful for the concrete description of the architecture and integrity of the neural tracts.5,6
In the current study, we used DTT to investigate a patient with RE and to examine the detailed characteristics of a splenial lesion of the corpus callosum.
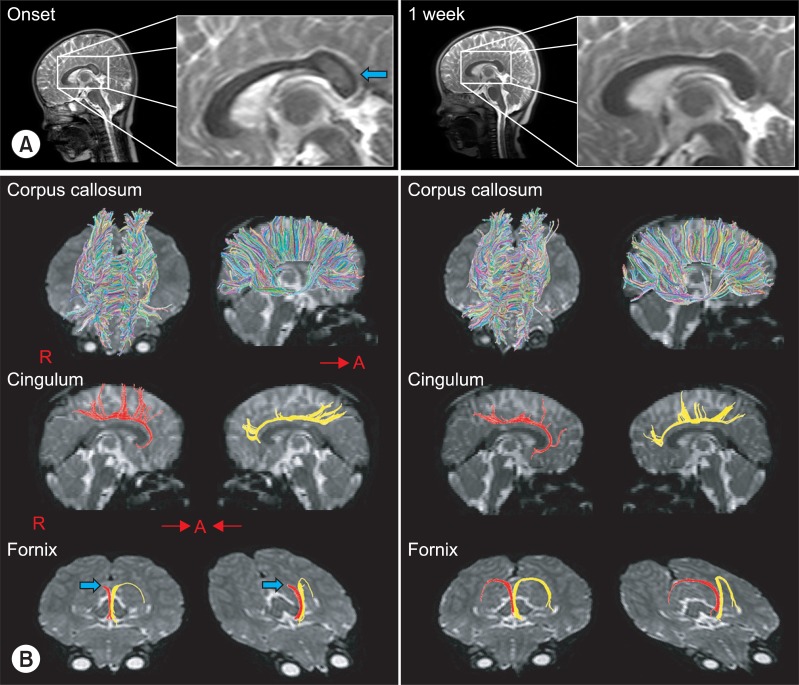
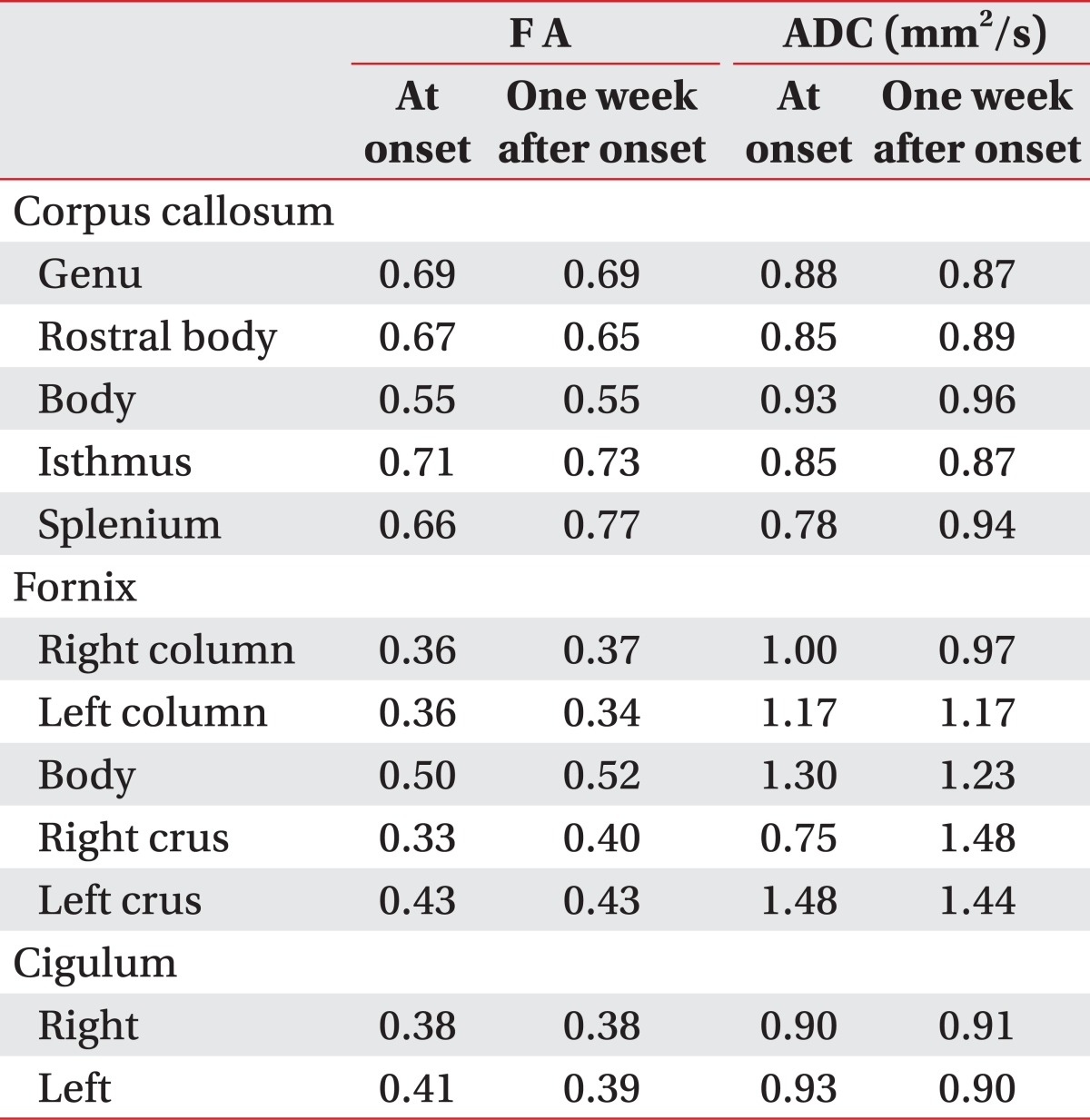
A 30-month-old female was brought to the emergency department after a brief (<5 minutes) generalized convulsive seizure. The patient had displayed a fever for the 3 days before admission, had vomited approximately 15 times, and had experienced 2 or 3 episodes of watery diarrhea on the day of admission. Before arrival at the emergency department, the patient experienced an episode of shaking of the arms and legs, with the eyes rolling upward. The witnesses' descriptions were consistent with a generalized tonic-clonic seizure. The patient's past medical history, family history, growth history, and developmental history were otherwise unremarkable. The patient's temperature was 37.5℃, heart rate 110, respiratory rate 28, weight 13.1 kg (50-75th percentile) and length 96.7 cm (95-97th percentile). The patient exhibited a drowsy mental status, but other neurological examinations did not reveal other defined abnormal findings. The patient underwent extensive diagnostic evaluations. The results of lab tests performed on admission were positive for rotavirus antigen in stool. Laboratory findings included a WBC count of 12,600/µl and platelet count of 905,000/µl; both were mildly elevated from normal. There were non-specific findings on all other laboratory tests, including a comprehensive metabolic panel, spinal fluid, blood culture, and urine culture. The patient was diagnosed with RE by a pediatric neurologist. The patient received effective replacement of fluids and electrolytes. Anticonvulsant medication was not taken without recurrent seizures. After one week, the patient displayed a much improved condition and was discharged 2 weeks after onset. Brain MRI, including DTI, was performed at onset and one week after onset. Lesions were well defined and ovoid in the SCC. The SCC lesion was homogeneously hyperintense on T2-weighted images and isointense or slightly hypointense on T1-weighted images; the lesion was not detected in MRI conducted one week later (Fig. 1). DTI data were acquired twice simultaneously with a conventional MRI (onset and one week after onset) using a 1.5-T Philips Gyroscan Intera system equipped with a multi-channel head coil with a single-shot spin echo-planar imaging sequence. For each of the 32 non-collinear and non-coplanar diffusion-sensitizing gradients, 60 contiguous slices were acquired parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows: matrix=128×128 matrix; field of view=221×221 mm2; TE=76 ms; TR=10,726 ms; SENSE factor=2; EPI factor=49; b=1,000 mm2 s-1; NEX=1; and a slice thickness of 2.3 mm. Eddy current image distortions and motion artifacts were removed using affine multiscale 2-dimensional registration, which was performed using the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl). The analysis for callosal projections was conducted using a single region of interest (ROI) approach using DTI-studio software CMRM (Johns Hopkins Medical Institute, Baltimore, USA). A callosal ROI was defined manually at the red portion of the midsagittal plane on the color map. Additional fiber tracking for the fornix and the cingulum were identified using fibers passing through two ROIs on the color map. The seed ROI of the fornix was placed on the junction between the column and body, and the target ROI was the junction between the body and crus. For the cingulum, the seed ROI was located in the green portion of the anterior cingulum areas and the target ROI was in the green portion of the posterior cingulum areas on the coronal slice.6 Fiber tracking was initiated at the center of a seed voxel with a fractional anisotropy (FA) >0.2 and ended at a voxel with a fiber assignment of FA <0.2 and a tract turning-angle of <60 degrees. Also, the FA values of cingulum, corpus callosum and fornix were measured. The whole corpus callosum was divided into 5 regions (genu, rostral body, body, isthmus and splenium) according to a well-established protocol, with 7 subdivisions of the corpus callosum,7 and FA and apparent diffusion coefficient (ADC) values of each of the 5 regions of the corpus callosum were measured. We evaluated the FA and ADC values of 3 divided regions of the fornix (column, body and crus) (Table 1).
The initial FA value of SCC at onset (0.66) was noticeably lower than that of follow-up FA value at one week after onset (0.77). However, the other regions of the corpus callosum showed no definite differences in FA values between the initial and follow-up data. For the fornix, only the region of the right crus had prominent interval changes between initial (0.33) and follow-up value (0.40). The cingulum showed no definite differences between initial and follow-up data. The initial ADC value was decreased and the follow-up value was increased, in the SCC and right fornical crus regions. For the other regions, there were no definite differences between initial and follow-up ADC values. The results of initial DTT demonstrated spared integrity of the corpus callosum, although a splenial lesion was apparent on conventional MRI. However, fornix revealed discontinuity of the right fornical crus. On the follow-up DTT at one week after onset, the interrupted right fonical crus was restored. In the cingulum, there were no definite abnormal findings at onset or at follow-up DTT (Table 1, Fig. 1).
In the current case study, we observed changes in DTT along with clinical changes in a patient with RE. At the time of the first DTI scan, this patient had a generalized convulsive seizure and appeared drowsy. On DTT, it revealed a decreased FA value, with spared integrity of the SCC, although a definite encephalomalatic lesion in the SCC was visible on conventional MRI. Additionally, he showed cognitive impairment on the day of admission. On DTT, the fornix showed not only disrupted integrity, but decreased FA value of the right crus. After treatment, the patient recovered to normal alertness and awareness, and theses clinical changes area well correlate with DTT changes. At the time of follow-up conventional MRI, DTI and DTT revealed normal findings of the fornix and corpus callosum.
Salmi et al.1 was the first to describe central nervous system involvement with a rotavirus. The 1978 study reported on 2 children with RE. The first case developed a fatal Reye's syndrome and the other patient showed symptoms of encephalitis with slow recovery. In 2009, a neuroimaging study about RE described a 2-year-old boy who experienced persistant diarrhea, vomiting, and a sudden disturbance of consciousness. Initial brain diffusion-weighted MRI demonstrated high signal intensity on the SCC, but the splenial signal returned to normal within 6 days as the disturbed consciousness improved.2 The clinical features and neuroimaging findings of the current study are very similar to those of these previous studies. The most likely mechanisms for the reversible SCC lesion in RE was proposed by Tada et al.3 They posited that the reversible SCC lesions may be related to the transiently decreased ADC values of the lesions: intramyelinic edema from separation of myelin layers, or an influx of inflammatory cells and macromoecules. Furthermore, they postulated that the isolated involvement of SCC is from a different arterial supply from the vertebrobasilar system, contrary to other parts of the corpus callosum supplied by the carotid system, which has special affinity for the receptors on splenial axons or surrounding myelin sheaths to viral antigens or receptors on the antibodies induced by the antigen, resulting in increased vulnerability of the SCC.3,8
Although the pathophysiology of delirium is not well established, delirium in RE is related to both cerebral hemispheres and connections of both hemispheres, rather than one hemisphere. The causes of delirium are intoxication, fever or encephalitis and generalized disruption of higher cognitive function may develop in delirium. The SCC connects the bilateral occipital and temporal lobes and is closely related to the limbic system. Therefore, encephalomalatic lesion in the SCC revealed by decreased FA value on DTI may lead to the disconnection of both temporal and occipital lobes and development of higher cognitive dysfunction, thus resulting in the altered behavior.4 Moreover, the authors presumed that not only splenial lesion of the corpus callosum, but also other microstructural pathophysiological mechanisms around corpus callosum may result in abnormal neurologic symptoms.
The fornix is an important component of the Papez circuit and limbic system. It connects the hippocampus and prefrontal cortex and plays an important role in cognitive function, such as intelligence, visuospatial memory, overall episodic memory, and affect.6,9,10 Consequently, fornical injury may lead to significant impairment of cognitive function in various diseases. In 2010, Zhuang et al.10 investigated the correlation between fornix and cognitive function using DTI in 165 mild cognitive impairment patients; the best discrimination between mild cognitive impairment patients and normal controls was achieved by combining FA measures for the SCC and those of the fornical crus. Another study examined a patient with diffuse axonal injury using DTT. Their results revealed that both cingulum and fornix showed severe degeneration as his memory impairment had been aggravated.6 As the fornix was disrupted on right crus on the first DTT in our study, the authors proposed that patients who demonstrated severe cognitive impairment and delirious behavior as sequelae of RE might to the result of both a splenial lesion and a fornix injury.
In addition, we used the diffusion parameter and DTT for the detailed microstructural evaluation of white matter lesion. The FA value is the most widely used DTI parameter and represents the degree of directionality of microstructures (eg, axons, myelin and microtubules). Reduced FA values of the SCC and fornical crus appear to be related to disintegration of neuronal fibers. However, the corpus callosum is the largest and main fiber tract for cerebral hemispheric transfer with at least 200 million nerve fibers. On the contrary, the fornix is a long and thin structure and the crus is located close to the posterior portion of the corpus callosum, and probably appears to be more vulnerable to rota virus infection than the corpus callosum; however, it is very difficult to assess this by conventional MRI due to the deep location within the brain. The authors suggest that this may be the reason for different results for integrity of corpus callosum and fornix in DTT.
In conclusion, we demonstrate that DTT can be a useful modality to assess microstructural lesions in a patient with RE that is related not only to SCC injury, but also to fornical injury. To the best of our knowledge, there has been no study about microstructural pathology using DTT in patients with RE. However, it is unclear whether this observation is an epiphenomenon or plays a more definite role in diagnosing RE as it is only a single case. Moreover, clinical functional evaluation, such as memory function, could not be obtained due to the patient's young age. Further complementary studies involving larger case numbers and detailed functional evaluation are warranted to corroborate these findings.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0015251).
References
1. Salmi TT, Arstila P, Koivikko A. Central nervous system involvement in patients with rotavirus gastroenteritis. Scand J Infect Dis. 1978; 10:29–31. PMID: 204984.

2. Fukuda S, Kishi K, Yasuda K, Sejima H, Yamaguchi S. Rotavirus-associated encephalopathy with a reversible splenial lesion. Pediatr Neurol. 2009; 40:131–133. PMID: 19135631.

3. Tada H, Takanashi J, Barkovich AJ, Oba H, Maeda M, Tsukahara H, Suzuki M, Yamamoto T, Shimono T, Ichiyama T, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004; 63:1854–1858. PMID: 15557501.

4. Takanashi J, Tada H, Kuroki H, Barkovich AJ. Delirious behavior in influenza is associated with a reversible splenial lesion. Brain Dev. 2009; 31:423–426. PMID: 18793826.

5. Malykhin N, Concha L, Seres P, Beaulieu C, Coupland NJ. Diffusion tensor imaging tractography and reliability analysis for limbic and paralimbic white matter tracts. Psychiatry Res. 2008; 164:132–142. PMID: 18945599.

6. Hong JH, Jang SH. Degeneration of cingulum and fornix in a patient with traumatic brain injury: diffuse tensor tractography study. J Rehabil Med. 2010; 42:979–981. PMID: 21031297.

7. Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989; 112:799–835. PMID: 2731030.
8. Bulakbasi N, Kocaoglu M, Tayfun C, Ucoz T. Tansient splenial lesion of the corpus callosum in clinically mild influenza-associated encephalitis/encephalopathy. Am J Neuroradiol. 2006; 27:1983–1986. PMID: 17032879.
9. Vachha B, Adams RC, Rollins NK. Limbic tract anomalies in pediatric myelomeningocele and Chiari II malformation: anatomic correlations with memory and learning--initial investigation. Radiology. 2006; 240:194–202. PMID: 16793979.

10. Zhuang L, Wen W, Zhu W, Trollor J, Kochan N, Crawford J, Reppermund S, Brodaty H, Sachdev P. White matter integrity in mild cognitive impairment: a tract-based spatial statistics study. Neuroimage. 2010; 53:16–25. PMID: 20595067.

Fig. 1
Results of conventional T2-weighted MRI and DTT. (A) T2-weighted images showed an encephalomalatic lesion (arrow) in the splenial lesion of the corpus callosum that disappeared in one week later follow-up MRI. (B) DTT at onset for the corpus callosum and cingulum showed no definite abnormal finding, although a splenal lesion was apparent on conventional MRI. However, the integrity of right fornical crus (arrow) was interrupted. A second DTT taken 1 week later from onset, integrity of the fornix was restored and well preserved. DTT of corpus callosum and cingulum findings were normal at follow up DTT. DTT: Diffusion tensor tractography.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download