Abstract
Objective
To find out the predictive value of the ΣΔST/ΔHR index for restenosis after percutaneous coronary intervention (PCI).
Method
Subjects of this research were patients who participated in a cardiac rehabilitation (CR) program as six to eight weeks of a hospital-based program after receiving PCI to treat acute coronary syndrome (ACS). The patients received coronary angiography (CAG) at the onset of the ACS and nine months after that, and also received an exercise tolerance test (ETT) at the start of the CR program and several days before receiving a follow-up CAG. In ETT, we used the sum of the ST depression (ΣΔST index) of leads II, III, aVF, V4-6 as well as the sum of the ΔST/ΔHR (heart rate) (ΣΔST/ΔHR index) in the same leads and the sum of the ΔST/ΔRPP (rate pressure product) (ΣΔST/ΔRPP index) in the same leads. We compared the predictive power of each index of ETT for restenosis after PCI.
Results
The sensitivity, specificity, positive predictive value, and negative predictive value of ΣΔST index were 69%, 47%, 31%, and 82%. The ΣΔST/ΔHR index was 13.7±5.2 in the restenosis group and 9.3±5.6 in the patent group (p=0.017). The sensitivity, specificity, positive predictive value, and negative predictive value of this index were 85%, 63%, 44%, and 92%. The ΣΔST/ΔRPP index were 0.10±0.08 in the restenosis group and 0.06±0.04 in the patent group (p=0.016). The sensitivity, specificity, positive predictive value, and negative predictive value of this index were 54%, 76%, 44%, and 83%.
Percutaneous coronary intervention (PCI) has been used to treat coronary artery disease (CAD).1 In contrast to the increasing experience with PCI, only a modest improvement has been gained in the control of restenosis. The latter is defined by many as the 'Achilles heel' of PTCA. It occurs in 15-50% of the patients after an initially successful PTCA, most commonly within 6 months of the procedure and after a symptom-free period.2 Coronary angiography (CAG) has become the leading method in detecting restenosis following PCI, but it is an expensive and invasive diagnostic tool. Using CAG has risk of perforation of the vessel, aortic dissection, hematoma, infection, thrombus or embolism.2
In contrast, an exercise tolerance test (ETT) is simple, cheap, noninvasive and readily available. But it has been reported that an ETT has a sensitivity of only 20% to 60% and a specificity of 80% to 90%.3 The heart rate (HR)-adjusted index of the ST-segment depression during ETT has been proposed as a more accurate electrocardiographic criterion for the diagnosis of CAD.4 But the heart rate (HR)-adjusted index of ST-segment depression at one lead does not sufficiently reflect myocardial ischemia in a patient with multivessel CAD.5 This is why some studies show that the sum of the ΔST/ΔHR index in leads II, III, aVF, V4, V5, V6 allows for more accurate prediction of restenosis after successful PCI in patients with multivessel CAD.5
Yet there is nearly no study about the usefulness of ETT to predict recurrent CAD. So we investigate the clinical usefulness of ETT in predicting restenosis which has performed 9 months after the initial acute stage of treatment and the cardiac rehabilitation program.
We developed useful criteria, criterion A are the sum of the changing rate of the ST segment at lead II, III, aVF, V4, V5, V6 (ΣΔST index); criterion B is the ΔHR adjusted index of the sum of ΔST at same leads (ΣΔST/ΔHR index); criterion C is the ΔRate pressure product (RPP) adjusted index of the sum of ΔST at the same leads. And we compared the usefulness of these criteria with each other in respect to predicting restenosis after a PCI.
We enrolled patients who were admitted to our cardiovascular center with acute coronary syndrome (ACS) between January 2007 and December 2008, or those who were referred to the cardiac rehabilitation clinic after undergoing a PCI. The patients participated in a 6-8 week hospital based exercise after discharge. Then they were advised to participate in a community-based self-exercise program. We enrolled patients that could be followed up for 9 months after ACS onset. Our study was a prospective cohort study. The exclusion criteria included patients with prior experience with PCI or CABG; a left ventricular ejection fraction (LVEF) of less than 40%, uncontrolled arrhythmia, hypertension, or diabetes; as well as other issues that would preclude a potential patient from participation in the program.
We followed 51 patients, and among these, the follow up CAG positive group included 14 patients and the CAG negative group included 38 patients. There was no significant difference in the gender distribution, mean age, rate of patients who were diagnosed with ST elevation myocardial infarction (MI) or non ST elevation MI or unstable angina (UA), stent insertion site distribution among the coronary artery segments, past history (which included hypertension, diabetes mellitus, and dyslipidemia), body mass index, LVEF at the initial cardiac rehabilitation (CR), or the sort of cardiac drugs between the two groups (Table 1).
Subject patients received CAG at the onset of ACS and nine months afterward. They also received ETT at the start of the CR program and several days before receiving a follow-up CAG. All of the subject patients had been informed about the purpose, method and potent complication of a CAG and ETT before these procedures. Consent was received from the study subjects prior to beginning the study and then we proceeded with the study.
The CAG was conducted in the following fashion. A standard image acquisition was performed using ≥2 angiographic projections of the stenosis. Intracoronary nitroglycerin was administered to provide maximum coronary vasodilation. At follow-up, a repetition of the identical angiographic projections of the lesion was performed. The lesion length was defined as the axial extent of the lesion that contained a shoulder-to-shoulder lumen reduction by 20%. Selected images for the analysis were identified by the use of angiographic projections that demonstrated stenosis in an unforeshortened view, minimized by the degree of vessel overlap, and displayed stenosis in its "sharpest and tightest" view. The target lesion was defined as the stented segment plus a 5 mm segment proximal and distal to the stent or progression of the native coronary vessel. Angiographic follow-up was routinely performed at 6-9 months after the index procedure unless an earlier angiography was required for clinical reasons. At 6-9 months, angiographic restenosis was measured as an in-segment late loss of the stented coronary artery in addition to newly developed native coronary stenosis via quantitative coronary angiography using CAAS 5.9 (Pie Medical Imaging B.V., Maastrichitt, Netherlands).
We defined angiographic positive cases as the binary angiographic restenosis which was defined as either the progression of a percent diameter stenosis up to 50% or more or a newly developed native coronary stenosis of 75% or more at the qualifying angiographic follow-up. The late luminal loss per stent was defined as the minimal luminal diameter (MLD) immediately after the procedure minus the MLD at a 6-9 month follow-up. Two experienced cardiologists independently interpreted the images blinded. Disagreement was resolved by reanalysis and consensus.
Subject patients received ETT at the start of the CR and at nine months after that. A real-time recording 12-channel ECG Q4500 (Quinton Instrument Co., Boston, USA), a respiratory gas analyzer QMC (Quinton Instrument CO., Boston, USA), an automatic blood pressure and pulse monitor Model 412 (Quinton Instrument Co., Boston, USA), and a treadmill Medtrack ST 55 (Quinton Instrument Co., Boston, USA) were included in the test. The VO2max was measured with a respiratory gas analyzer, and the maximum heart rate, the stable heart rate, and the myocardial oxygen demand (MVO2) were estimated using an ECG, and an automatic blood pressure and pulse monitor. The MVO2 was calculated by multiplying the systolic blood pressure and heart rate as the rate pressure product (RPP). The submaximal MVO2 was measured at the end of stage 3 of the modified Bruce protocol.
We evaluated the usefulness of the following parameters in predicting restenosis. Based on the results of ETT, we measured: criterion A as the sum of the changing rate of the ST segment at lead II, III, aVF, V4, V5, V6 (ΣΔST index); criterion B as the sum of the ΔHR adjusted index of ΔST at the same leads (ΣΔST/ΔHR index); criterion C as the sum of the ΔRate pressure product (RPP) adjusted index of ΔST at same leads (ΣΔST/ΔRPP index). We compared these criteria with the results of follow up CAGs. We then identified the predictive power of each index of ETT for restenosis after PCI.
The data was statistically analyzed with SAS Enterprise Guide 4.1 (4.1.0.471). The Wilcoxon rank sum test was used to compare gender, age, the rate of patients who were diagnosed as ST elevation myocardial infarction (MI) or non ST elevation MI or unstable angina (UA) with a past history which included hypertension, diabetes mellitus, dyslipidemia, body mass index, LVEF at the initial cardiac rehabilitation (CR), a sort of drug between the positive CAG group and the negative CAG group. The Wilcoxon signed rank test was used to compare the criterion for A, B, C in each group at baseline and 9 months. It was also used to compare the resting heart rate (HR), the maximal HR, the VO2max, the maximal and the submaximal myocardial oxygen demand (MVO2) in each group at baseline and 9 months. A p-value of <0.05 was considered statistically significant.
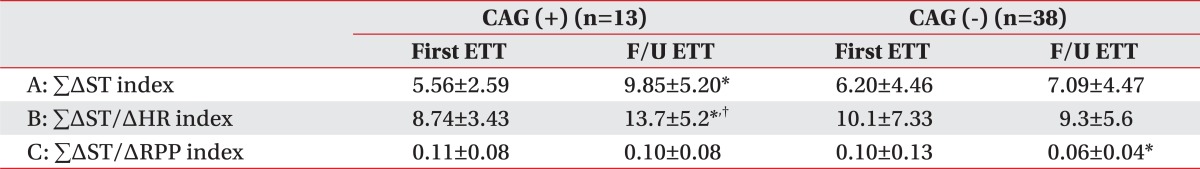
In the negative CAG group, criterion A at baseline and 9 months was not statistically significant. However, in the positive CAG group, criterion A was 5.56±2.59 at baseline and increased to 9.85±5.20 at 9 months. It was statistically significant (p<0.05) (Table 2).
In the negative CAG group, criterion B was 10.1±7.33 at baseline and decreased 9.3±5.6 at 9 months. It was not statistically significant. However, in the positive CAG group, criterion B was 8.74±3.43 at baseline and increased to 13.7±5.20 at 9 months. It was statistically significant (p<0.05) (Table 2).
In the negative CAG group, criterion C was 0.10±0.13 at baseline and decreased 0.06±0.04 at 9 months. And it was statistically significant (p<0.05). However, in the positive CAG group, criterion C was 0.11±0.08 at baseline and decreased to 0.10±0.08 at 9 months. It was not statistically significant (Table 2).
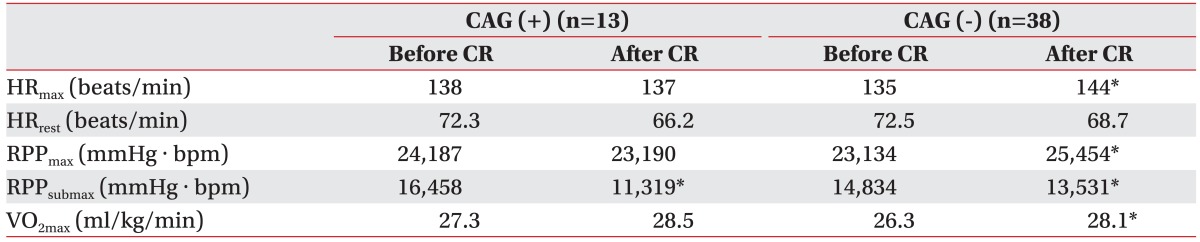
There was a significant difference in the submaximal rate pressure product (RPPsubmax) between the two groups. However, the maximal HR, the maximal RPP and the VO2max increased more significantly in the negative CAG group at baseline and 9 months than they did in the positive CAG group (p<0.05) (Table 3).
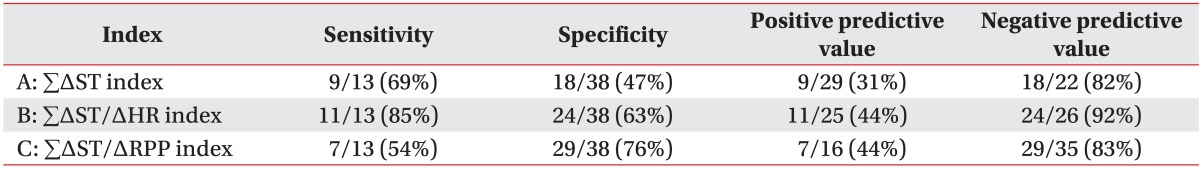
When we used criterion A, this criterion increased at follow-up compared with the value obtained at the start of CR in 9 of the 13 patients in the positive CAG group and in 18 of the 38 in the negative CAG group. The sensitivity of the increase in criterion A at follow-up for predicting restenosis was 69%, specificity was 47%, positive predictive value was 31%, and the negative predictive value was 82% (Table 4).
Based on criterion B, 11 of the 13 patients in the positive CAG group and 24 of the 38 patients in the negative CAG group had a positive ETT at follow up. Sensitivity of the increase in criterion B at follow-up for predicting restenosis was 85%, specificity was 63%, positive predictive value was 44%, and negative predictive value was 92% (Table 4).
Based on criterion C, 7 of the 13 patients in the positive CAG group and 29 of the 38 patients in the negative CAG group had positive ETT at follow up. The sensitivity of the increase in criterion C at follow-up for predicting restenosis was 54%, and the specificity was 76%. This criterion had the highest specificity in the three criterions. The positive predictive value was 44%, and negative predictive value was 83% (Table 4).
Coronary artery disease has high prevalence and high mortality rates all over the world. Due to advances in medical technology, the mortality rate seems to have diminished, but the prevalence rate has gradually increased.6,7 However restenosis occurs in 15-50% of patients after an initially successful PTCA, most commonly within 6-9 months of the procedure and usually after a symptom-free period.2 For that reason, we need active prevention treatment of CAD recurrence and a precise early diagnostic tool of CAD restenosis in any case.
Currently, the three main noninvasive tests suggest the presence of coronary artery restenosis: ETT, myocardial perfusion imaging (MPI) and stress echocardiography (s-echo). Based on the meta-analysis, sensitivity of the prediction of coronary artery restenosis was 54% in ETT, 83% in MPI, and 82% in s-echo. The specificity was 70% in ETT, 79% in MPI, and 86% in s-echo. The positive predictive value was 64% in ETT, 80% in MPI, and 80% in s-echo. The negative predictive value was 61% in ETT, 85% in MPI, and 79% in s-echo. The usefulness of the prediction for restenosis was lowest in ETT. In this manner, the conventional ETT had a lower sensitivity to predict restenosis, but ETT had been used frequently in CR clinics for various purposes including the confirmation of a cardiac response after exercise stimulation, evaluation of exercise capacity, evidence of exercise prescription, among other criteria. Thus our study attempts to consider ETT within the framework of the prediction for restenosis.
Until now, there has been sufficient efforts to complement the lower sensitivity of ETT for predicting restenosis in many studies.8,9 A study by Koide et al.3 showed that the sensitivity and specificity for the difference between the maximum ST-segment depression before and after PCI were 77% and 76%, the sensitivity and specificity for the difference between the sum of ST-segment depression at all leads before and 3 months after PCI were 77% and 83%. So they reported that these indexes increased the accuracy of diagnosing restenosis compared to simple ETT.3
Based on our study, criterion B (ΣΔST/ΔHR) had the highest sensitivity (85%) among criterion A, B, and C for predicting restenosis after PCI, and criterion C (ΣΔST/ΔRPP) had the highest specificity (76%). Although the specificity of criterion B (ΣΔST/ΔHR) was not high (63%), its negative predictive value was high (92%). Criterion B (ΣΔST/ΔHR) had a higher sensitivity, specificity, positive predictive value and negative predictive value as compared to criterion A (ΣΔST), and criterion B (ΣΔST/ΔHR) which was more useful in predicting restenosis in clinics. It is possible to complement specificity while using criterion C (ΣΔST/ΔRPP) together which had a high specificity. Also, in the case of negative ETT results, these criterions can be applied to patients that were suspected of having restenosis. There is also the benefit of predicting early restenosis progression by checking the change in criteria as compared to the previous results.
Criterion A significantly increased in the positive CAG group 9 months after CR, but criterion C which is the RPP adjusted of criterion A (ΣΔST) decreased significantly in the negative CAG group. It means that RPP had decreased more at 9 months after CR than after PCI. Furthermore, criterion C (ΣΔST/ΔRPP index) can be helpful in predicting restenosis, to estimate myocardial burden and ischemia during exercise as well as to see the improvement of a patient's cardiopulmonary capacity.
Aerobic exercise in CR can reduce the myocardial burden when performing daily physical activities because aerobic exercise increases the VO2max and decreases the submaximal heart rate. In addition, the VO2 in the systemic muscles may be reduced, and as these muscles are needed to exercise at the same intensity, reduction in the VO2 lessens the myocardial burden in patients with heart disease. The incidence of myocardial ischemia can be reduced by decreasing the MVO2 because myocardial ischemia generally occurs at the same RPP.10 Even in this study, submaximal RPP decreased in both the positive CAG group and the negative CAG group. Consequently, the relieved patients' RPP may be expected to enhance cardiopulmonary function and decrease ischemia in daily and socio-occupational activities. The VO2max increased in both the positive and negative CAG group, but statistically significant increases were only observed in the negative CAG group. It seems indirectly that a patient who has an increased VO2max significantly lessen the progression of coronary artery stenosis.
There are some limitations in our study. This is not a randomized comparative study. We enrolled only patients who participated in CR, so that it is difficult to accept general results in all patient who received PCI. Further for all patients who took PCI, long-term follow-up studies will be required in the future.
The criterion B (ΣΔST/ΔHR index) would be a useful diagnostic tool to increase the accuracy of prediction for restenosis after PCI compared to ETT. We recommend the use of the criterion C (ΣΔST/ΔRPP) with criterion B as it will be more useful in predicting the myocardial burden and ischemia, and in identifying the improvement of cardiopulmonary exercise capacity.
References
1. Grüntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979; 301:61–68. PMID: 449946.
2. Dori G, Denekamp Y, Fishman S, Bitterman H. Exercise stress testing, myocardial perfusion imaging and stress echocardiography for detecting restenosis after successful percutaneous transluminal coronary angioplasty: a review of performance. J Intern Med. 2003; 253:253–262. PMID: 12603492.

3. Koide Y, Yotsukura M, Ando H, Yoshino H, Ishikawa K. Accuracy of treadmill exercise electrocardiography in detecting restenosis following single-vessel percutaneous transluminal coronary angioplasty. Am J Cardiol. 1997; 80:1282–1286. PMID: 9388099.

4. Chalela WA, Kreling JC, Falcao AM, Hueb W, Moffa PJ, Pereyra PL, Ramires JA. Exercise stress testing before and after successful multivessel percutaneous transluminal coronary angioplasty. Braz J Med Biol Res. 2006; 39:475–482. PMID: 16612470.

5. Hamasaki S, Abematsu H, Arima S, Tahara M, Kihara K, Shono H, Nakao S, Tanaka H. A new predictor of restenosis after successful percutaneous transluminal coronary angioplasty in patients with multivessel coronary artery disease. Am J Cardiol. 1997; 80:411–415. PMID: 9285650.

6. Lane D, Carroll D, Ring C, Beevers DG, Lip GY. Predictors of attendance at cardiac rehabilitation after myocardial infarction. J Psychosom Res. 2001; 51:497–501. PMID: 11602219.

7. Blumenthal JA, Williams RS, Wallace AG, Williams RB Jr, Needles TL. Physiological and psychological variables predict compliance to prescribed exercise therapy in patients recovering from myocardial infarction. Psychosom Med. 1982; 44:519–527. PMID: 7163455.

8. Desmet W, de Scheerder I, Piessens J. Limited value of exercise testing in the detection of silent restenosis after successful coronary angioplasty. Am Heart J. 1995; 129:452–459. PMID: 7872170.

9. Schroeder E, Marchandise B, de Coster P, Brichant C, Mitri K, Pieters D, Kremer R. Detection of restenosis after coronary angioplasty for single vessel disease: how reliable are exercise electrocardiography and scintigraphy in asymptomatic patients? Eur Heart J. 1989; 10(Suppl G):18–21. PMID: 2627944.
10. Kim C, Youn JE, Choi HE. The effect of a self exercise program in cardiac rehabilitation for patients with coronary artery disease. Ann Rehabil Med. 2011; 35:381–387. PMID: 22506148.

Table 1
Characteristics of Subjects
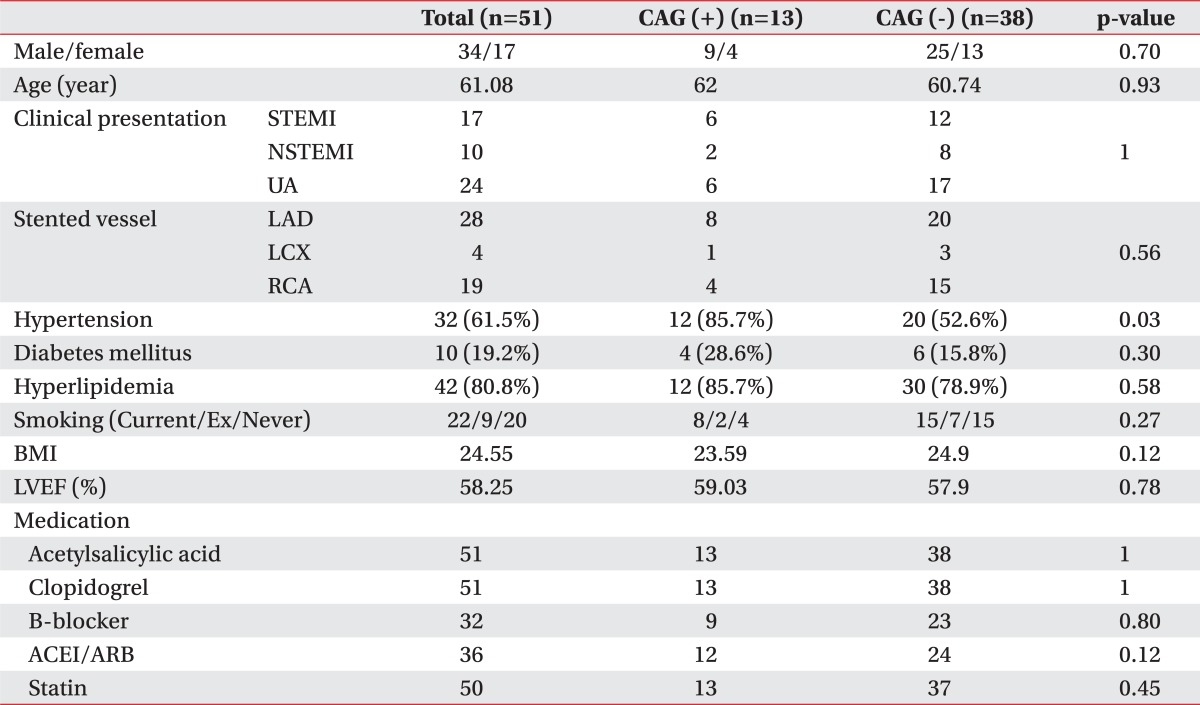
CAG: Coronary angiography, STEMI: ST elevation myocardial infarction, NSTEMI: Non ST elevation myocardial infarction, UA: Unstable angina, BMI: Body mass index, LVEF: Left ventricular ejection fraction, LAD: Left anterior descending, LCX: Left circumflex, RCA: Right coronary artery, ACEI: Angiotensin converting enzyme inhibitor, ARB: Angiotensin receptor blocker
Table 2
Comparison of Indices between the Positive Angiographic Group and the Negative Angiographic Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download