Abstract
Objective
To investigate whether the cartilage regenerative effects of intra-aricular platelet-rich plasma (PRP) are different, according to the severity of osteoarthritis (OA), in a collagenase-induced knee OA rabbit model.
Method
New Zealand white rabbits (N=21) were randomly divided into three groups. Three different doses (0.25 mg, group 1; 0.5 mg, group 2; and 1.0 mg, group 3) of collagenase were injected twice into both knees of each group under an ultrasound guidance. The mean platelet concentration of the PRP fraction was 2,664±970×103/µl and was enriched 8.2-times, compared with the whole blood. PRP (0.3 ml) was injected into the left knee and saline (0.3 ml) into the right knee at 4 weeks, and macroscopic and histological scores of both injected knees were evaluated at 9 weeks after the first collagenase injection.
Results
Macroscopic and histological scores of group 3 were significantly higher than those of group 1 and 2 (p<0.05). Macroscopic and histological scores of the PRP-injected knees were significantly lower than those of the saline-injected knees, in all groups (p<0.05). Differences of gross morphologic and histologic scores between saline- and PRP-injected knees in group 3 were significantly higher than those in group 1 and 2 (p<0.05).
Conclusion
Intra-articular PRP injection influences cartilage regeneration in all severities of rabbit knee OA, and the cartilage regenerative power of PRP injection in moderate knee OA was greater than that in mild or very mild OA. A large preclinical trial is needed to establish the validity of our study.
Osteoarthritis (OA) results from articular cartilage loss, induced by a complex interaction of genetic, metabolic, biochemical, and biomechanical factors with the secondary components of inflammation.1 Conservative treatments for OA include weight loss, therapeutic exercise, activity modification, assistive devices, oral medications, including acetaminophen, non-steroidal anti-inflammatory drugs, opioids, and intra-articular corticosteroid and hyaluronic acid (HA) injections.2 This is especially true in the elderly, the major targeted population for OA treatment, for whom one must consider the risk of upper gastrointestinal, cardiovascular, or renal adverse effects, and the diverse array of concomitantly used medications. Intra-articular injection of corticosteroid, a potent anti-inflammatory agent, is also limited for clinical practice because of articular cartilage damage, secondary infection, and systemic side effects with repeated injections.3 Intra-articular HA injection has been recognized as a safe and efficacious alternative therapy to corticosteroid injection, and is currently considered only as a disease-modifying treatment for knee OA.4 In addition, the efficacy of HA injection is dependent on the severity of OA. While HA injection is responsive in mild-to-moderate OA, it is not effective in OA with considerable effusion or gross architectural changes.5
Platelet contains an abundance of growth factors and cytokines that are crucial in the healing process of soft tissues and bone mineralization.6 Platelet also discharges many bioactive proteins responsible for attracting macrophages, mesenchymal stem cells, and osteoblasts, which not only promote scavenging of necrotic tissue but also facilitate tissue regeneration and healing.7 Platelet-rich plasma (PRP) is composed of 3-8-fold greater concentration of platelets, as compared to whole blood, and contains a hyper-physiological content of autologous growth factors. Chondrogenesis was demonstrated in the model of rabbit knee cartilage defects when PRP was used with a scaffold.8 With regard to cartilage regeneration, growth factors appear to have chondroinductive effects. Among platelet derived growth factors, transforming growth factor-β contributes to chondrocyte phenotype expression and mesenchymal stem cell chondrogenic differentiation, insulin growth factor to anabolic properties in cartilage regeneration, and platelet derived growth factor to chondrocyte proliferation and proteoglycan synthesis.9
Considering these regenerative effects of PRP on cartilage damage, we hypothesized that intra-articular PPR injection would be an effective treatment for collagenase-induced knee OA in a rabbit model. We presently report that the degree of cartilage degeneration is directly proportional to the collagenase injection dosage in the knee of rabbits, and the cartilage regenerative effect of intra-aricular PRP injection differs according to the severity of knee OA in the rabbit model.
Male New Zealand, 12-week-old, white rabbits (n=21) were housed in separate metal cages at a temperature of 23±2℃ and a relative humidity of 45±10%. They were provided free access to tap water and were fed a commercial rabbit diet. All animals did not get additional exercise and were allowed for normal activities in a cage with dimensions of 65×45×30 cm. There is no exercise protocol during the study period. Animal experiments were performed in accordance with the internationally accredited guidelines, and have been approved by the author's institutional Animal Care and Use Committee.
Anesthesia was induced with isoflurane (JW Pharmaceutical, Goyang, Korea) vaporized in oxygen and delivered via a large animal circle system. Both knee joints of all rabbits were shaved and sterilized, and three different doses (0.25 mg, 0.5 mg, and 1.0 mg) of collagenase type II from Clostridium histolyticum (Sigma-Aldrich, St. Louis, USA) were prepared for an intra-articular knee injection. Collagenase was dissolved in a sterile phosphate buffered saline (pH 7.4), and was filtered through a 0.22 µm membrane. The 21 rabbits were equally divided into three groups of seven. Injections of 0.25 mg, 0.5 mg, and 1.0 mg collagenase were delivered to both knees of group 1, 2, and 3, respectively. All injections were conducted by a physiatrist, using a commercially available ultrasound system E-CUBE 9® 3-12 MHz multi-frequency linear transducer (Alpionion Medical Systems, Seoul, Korea) (Fig. 1). The second injection with the same collagenase dose was repeated 3 days later (Fig. 2).10,11
For PRP preparation, 6.0 ml venous blood was drawn, using an aseptic technique from the marginal auricular vein and mixed with 2 ml of 0.129-mol/L sodium citrate in a sPRP system® PRP device (Huons, Goyang, Korea). It was centrifuged for 3 minutes at 3,200 rpm. The three layers that formed were comprised of platelet poor plasma, PRP, and red blood cells. The PRP layer was extracted through a special port and 0.3 ml was used for the injections. The number of platelets in the whole blood and isolated PRP fraction was assessed, using the Vet abc Plus hematology analyzer (Scil Animal Care, IL, USA). The mean platelet concentration of the whole blood was 324±25×103/µl (range: 277-356×103/µl). The mean platelet concentration of the PRP fraction was 2,664±970×103/µl (range: 2,005-3,120×103/µl), and represented an 8.2-fold increase over the concentration in the whole blood.
PRP (0.3 ml) was injected into the left knee and saline (0.3 ml) into the right knee, in rabbits in the three groups under ultrasound guidance, 4 weeks after the first collagenase injection.11,12 All procedures were performed under general anesthesia and sterile conditions. No medication was administered after the injection. The rabbits were euthanized by CO inhalation at 9 weeks after the first collagenase injection (Fig. 2).
Clinical observations were performed once daily in the afternoon. The rabbits were turned out on the ground of the area of 2 square meters, and their gait pattern was assessed by direct observation for 20 min, individually. In the intact limb, the knee and ankle angles went through a typical flexion and extension cycle during hoping of the rabbit. The lameness was defined as non-weight bearing of the affected limb and losing of typical flexion and extension cycle during hoping in comparison with the unaffected limb. The severity of the lameness was not quantified. The times to normal ambulation without the non-weight bearing lameness of the affected limb were recorded, and the lameness periods were calculated for each group. Two independent physiatrists, without prior knowledge of the experimental groups, performed the observation.
The knee joints were dissected after euthanasia and the lateral femoral condyle, which was the most destructive portion by collagenase in the knee, was examined for gross morphologic changes.10 Cartilage degeneration was assessed and measured using the Yoshimi scoring system (0, normal cartilage; 1, softened cartilage; 2, fibrillation; 3, erosion; 4, ulceration; and 5, loss of cartilage).13
The lateral femoral condyle was fixed with 10% neutral buffered formalin and decalcified with 20% ethylenediaminetetraacetic acid. The calcified condyle was embedded in paraffin and the standard frontal microsections with 5 µm width were prepared and stained with hematoxylin and eosin. If the stain was not proper, the next cartilage surface of the specimen was cut and stained. The degree of the cartilage degradation was assessed using the scoring system modified by Mankin et al.14 Histological evidence of cartilage degeneration was evaluated by the structural change of articular cartilage (0, normal; 1, surface irregularities; 2, pannus and surface irregularities; 3, clefts to transitional zones; 4, clefts to radial zones; 5, clefts to calcified zones; and 6, complete disorganization) and the cell status (0, normal; 1, diffuse hypercellularity; 2, cloning; and 3, hypocellularity). Total cartilage degeneration score ranged from 0 (normal) to 9 (complete disorganization and hypocellularity of articular cartilage). All cartilage sections were graded by a pathologist who was kept unaware of the treatment.
Statistical analyses were performed using the SPSS V. 14.0 software (SPSS, Chicago, USA). Kruskal-Wallis test was used to compare the gross and histological changes of cartilage among three groups with saline injection, and the difference of gross morphologic and histologic scores between saline- and PRP-injected knees among the three groups. Wilcoxon signed rank test was used to assess the differences between both knees injected with PRP and saline in each group. p-value<0.05 was considered statistically significant.
The average time to recovery of normal ambulation was 14±2 days in group 1 rabbits, 16±3 days in group 2 rabbits, and 20±3 days in group 3 rabbits. The average time to recovery of normal ambulation of group 3 was significantly longer than that of group 1 and 2 (p<0.05).
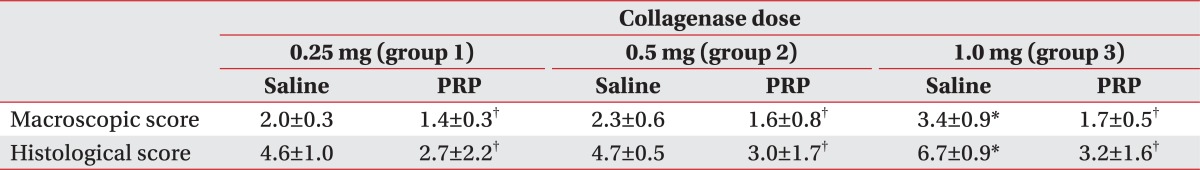
The severity of cartilage degeneration at the lateral femoral condyle was proportional to the increase of the injection dose of collagenase. Macroscopic and histological scores in group 3 were significantly higher than in groups 1 and 2 (p<0.05) (Table 1). However, there was no difference of scores between groups 1 and 2. The abnormal gross morphologic and histologic changes, such as the fibrillation and irregularity of cartilage surfaces, disappearance of surface-layer cells, and slightly diffuse cell growth in the transitional and radial zones were observed in group 1 (Fig. 3-A, D). Fibrillation of articular cartilage, and the cleft and cell cloning in the transitional and radial zones were noted in group 2 (Fig. 3-B, E). The ulceration of cartilage surface and loss and cell cloning in the radial zone were evident in group 3 (Fig. 3-C, F).
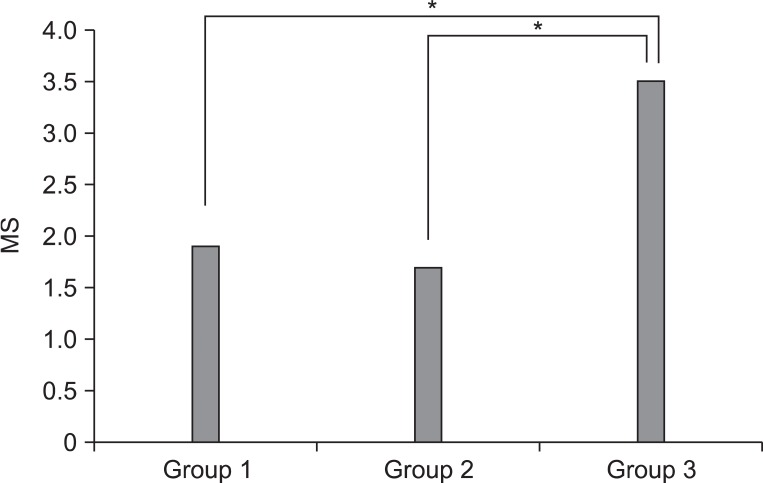
Macroscopic and histological scores of the PRP-injected knees were significantly lower than those of the saline-injected knees in all groups (p<0.05) (Table 1). The difference of gross morphologic and histologic scores between the saline and PRP-injected knees in group 3 was higher than those in groups 1 and 2 (p<0.05) (Fig. 4, 5). However, there was no significant difference between groups 1 and 2. Histologic examination showed nearly normal cartilage surfaces with normal cellularity in the transitional and radial zones in group 1 with PRP injection, irregularity of cartilage surface with normal cellularity in the transitional zone in group 2 with PRP injection, and a cleft in the transitional with apparent cloning of chondrocytes in the transitional zone in group 3 with PRP injection (Fig. 3-J, L).
Our study reveals that the difference of gross morphologic and histologic scores between the saline and PRP injected knees in group 3 was higher than those in groups 1 and 2, which suggests that PRP injection is more regenerative in the 1.0 mg collagenase-induced knee OA, like group 3, than the other groups. It is postulated that a comparatively lesser cartilage regeneration happened in groups 1 and 2 than in group 3, because the degree of cartilage degeneration in groups 1 and 2 was less than that in group 3, after an intra-articular collagenase injection. These findings are consistent with a previous study that the OA changes were induced by the intra-articular injection of different doses of collagenase, and were dependent on the injection doses.13 Additionally, the potent cartilage protective effects of several therapeutic regimens were proved in a 1.0 mg collagenase-induced osteoarthritis rabbit model. Therefore, we speculate that 1.0 mg collagenase injection is sufficient to induce reversible OA-like changes.
Some recent animal studies assessed the PRP effect on chondrogenesis and cartilage healing. In a rabbit model, 48 osteochondral defects, created in the femoropatellar groove, were divided into three groups: no treatment, treatment with autogenous PRP in poly-lacticglycolic acid, and with poly-lacticglycolic acid alone.8 The PRP treatment group demonstrated a greater extent of cartilage regeneration, as well as higher production of glycosaminoglycans in the extracelluar matrix. In addition, chondrogenesis was also verified in cartilage defects of the rabbit knee when PRP was used with a scaffold.15 Increased hyaluronic acid production and secretion were detected in synoviocytes from the patients with OA cultured in PRP. This suggests that intra-articular PRP injection could potentially serve as the endogenous source of chondroprotection and joint lubrication.16 Our results are similar to those of a previous study that reported PRP-mediated stimulation of either cell proliferation or matrix metabolism through articular chondrocytes in vitro.17 To the best of our knowledge, this is the first study to assess the possibility that the severity of knee OA may have an impact on the therapeutic response of intra-articular PRP injection.
Animal models of OA, in which an articular cartilage damage is induced with intra-articular collagenase injection, have proven to be similar to human OA.13 Presently, in a rabbit model of OA of the knee, the degree of articular cartilage degeneration at the lateral femoral condyle was proportional to the increase of intra-articular injection dose of collagenase. These findings are consistent with a previous study that the OA changes were induced by the intra-articular injection of 0.5-2.0 mg collagenase, and were dependent on the injection doses.13 In addition, presently, knee OA injected with PRP showed significantly lower gross and histological scores than those injected with saline, indicating that PRP injection facilitates the regeneration of damaged cartilage in collagenase-induced knee OA. There are several explanations for the healing effect of PRP injection in knee OA. First, several growth factors are usually stored in alpha-granules of platelets as the latent form. When platelets are activated, these factors are gradually released and participate in the regeneration process.18 Second, chondrocytes derived from the surrounding normal cartilage may be activated, and then migrate into the damaged cartilage. Third, mesenchymal stem cells or other multi-potent stem cells may also become activated and migrate to the cartilage lesion. This process is corroborated by a previous study that demonstrated the chondrogenic and proliferative effects of PRP on mesenchymal stem cells.19
The ideal concentration of platelets in PRP is not yet clear. Previous study demonstrated that the concentrations of growth factors in PRP were positively correlated with that of the platelet concentration of PRP.20 Qualitative and quantitative changes of the platelets may affect the regenerative power of PRP. In our study, PRP was prepared from the centrifugation of the autologous whole blood in rabbits, and the platelet concentration of the whole blood was compatible with the range of normal values for rabbit (158-650×103/µl).21 The platelet concentration of PRP was 8.2-times higher than that of the whole blood, thus, offering higher regenerative effects of PRP. Therefore, our PRP preparation is adequate to assess its therapeutic effects for cartilage regeneration because of the high platelet concentration.
Until now, intra-articular injection into the rabbit knee, under ultrasound guidance, has not been reported. In a human study, one third of the blind knee injections, performed by physician, were shown to be inaccurate.22 In our study, improvement of accuracy in the knee injection was achieved through an ultrasound guidance. A transducer was placed transversally on the anteriolateral portion of the rabbit knee, and intra-articular injection was performed when a needle was correctly placed in the knee joint on ultrasound guidance.
There are several limitations in our study. First, the small number of specimens made it difficult to determine the therapeutic effect of PRP in the knee OA of rabbits. Second, there was no control group for the comparison of the cartilage degeneration effects, according to the injection dose of collagenase, because specimens were not examined at 4 weeks after the first collagenase injection. Third, we did not conduct the histological examination of synovial changes in our study. The synovial membrane is also a source of proinflammatory and catabolic products, including metalloproteinases and aggrecanases, which contribute to articular matrix degradation. In the previous study, the inflammatory synovial reaction of the knee joint was the most severe at 1 week after collagenase injection. After then, the inflammatory responses in the synovial tissue lessened with time and weekly remained at 6 weeks after injection.13 In our study, PRP was injected into the knee at 4 weeks after the first collagenase injection, and the rabbits were euthanized at 9 weeks after the first injection. Therefore, it was difficult to decide whether the synovial changes were due to the effect of PRP or simply as the passing of time. Fourth, we did not consider the influence of physical performance of rabbit on cartilage regeneration. Fifth, we did not contemplate the influence experimental rabbit age since the degenerative processes progress, as time passed and better results are achieved in younger rabbits. Finally, we did not evaluate the therapeutic effects of PRP in the severe knee OA. Further studies will be needed to evaluate the PRP effects, according to the different platelet concentration, injection time, and number of injection in knee OA of rabbits, and in severe knee OA for the achievement of the best and more durable results.
Although our results are based on a relatively small number of specimens, the degree of cartilage degeneration was directly proportional to the collagenase injection dose in the knee OA of rabbits, and PRP injection demonstrated the effect of cartilage regeneration in all severity of rabbits' knee OA. In addition, the cartilage regenerative power of PRP injection in medium dose collagenase induced knee OA was greater than that in low doses. Further formal preclinical trial with more specimens is required to establish the validity of our study.
References
1. Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev. 2006; 7:239–250. PMID: 16866972.

2. Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995; 38:1541–1154. PMID: 7488273.
3. Goldspink DF, Goldberg AL. Influence of pituitary growth hormone on DNA synthesis in rat tissues. Am J Physiol. 1975; 228:302–309. PMID: 1147021.

4. Altman R, Brandt K, Hochberg M, Moskowitz R, Bellamy N, Bloch DA, Buckwalter J, Dougados M, Ehrlich G, Lequesne M, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis Cartilage. 1996; 4:217–243. PMID: 11048620.
5. Namiki O, Toyoshima H, Morisaki N. Therapeutic effect of intra-articular injection of high molecular weight hyaluronic acid on osteoarthritis of the knee. Int J Clin Pharmacol Ther Toxicol. 1982; 20:501–507. PMID: 7174151.
6. Anitua E, Sanchez M, Nurden AT, Nurden P, Orive G, Andia I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006; 24:227–234. PMID: 16540193.

7. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008; 1:165–174. PMID: 19468902.

8. Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010; 34:589–597. PMID: 19434411.

9. Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis Cartilage. 2006; 14:403–412. PMID: 16413799.

10. Kikuchi T, Sakuta T, Yamaguchi T. Intra-articular injection of collagenase induces experimental osteoarthritis in mature rabbits. Osteoarthritis Cartilage. 1998; 6:177–186. PMID: 9682784.

11. Kim SB, Kwon DR, Kwak H, Shin YB, Han HJ, Lee JH, Choi SH. Additive effects of intra-articular injection of growth hormone and hyaluronic acid in rabbit model of collagenase-induced osteoarthritis. J Korean Med Sci. 2010; 25:776–780. PMID: 20436717.

12. Huh JE, Baek YH, Lee JD, Choi DY, Park DS. Therapeutic effect of Siegesbeckia pubescens on cartilage protection in a rabbit collagenase-induced model of osteoarthritis. J Pharmacol Sci. 2008; 107:317–328. PMID: 18635922.

13. Yoshimi T, Kikuchi T, Obara T, Yamaguchi T, Sakakibara Y, Itoh H, Iwata H, Miura T. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop Relat Res. 1994; 298:296–304. PMID: 8118990.

14. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971; 53:523–537. PMID: 5580011.
15. Qi YY, Chen X, Jiang YZ, Cai HX, Wang LL, Song XH, Zou XH, Ouyang HW. Local delivery of autologous platelet in collagen matrix simulated in situ articular cartilage repair. Cell Transplant. 2009; 18:1161–1169. PMID: 19660173.

16. Anitua E, Sanchez M, Nurden AT, Zalduendo MM, de la Fuente M, Azofra J, Andia I. Platelet-released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology. 2007; 46:1769–1772. PMID: 17942474.

17. Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ, Lenz ME, Sah RL, Masuda K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006; 14:1272–1280. PMID: 16820306.

18. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004; 62:489–496. PMID: 15085519.

19. Drengk A, Zapf A, Sturmer EK, Sturmer KM, Frosch KH. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs. 2009; 189:317–326. PMID: 18689989.

20. Li M, Zhang C, Yuan T, Chen S, Lu R. Assessment study on a set of platelet-rich plasma preparation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011; 25:112–116. PMID: 21351624.
21. Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine. 2002. 2nd ed. Salt Lake: Academic Press.
22. Cunnington J, Marshall N, Hide G, Bracewell C, Isaacs J, Platt P, Kane D. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joint of patients with inflammatory arthritis. Arthritis Rheum. 2010; 62:1862–1869. PMID: 20222114.
Fig. 1
(A) Intra-articular injection was performed into a knee of rabbit under ultrasound guidance. (B) Longitudinal ultrasound image showed the needle (arrows) in the knee joint of rabbit.
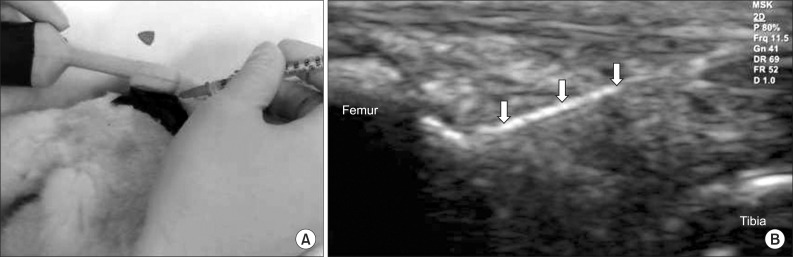
Fig. 2
Time line of collagenase, saline, and PRP injection. Both knees injections were performed twice with 0.25 mg (group 1), 0.5 mg (group 2), and 1.0 mg (group 3) collagenase on day 1 and 4, respectively. 0.3 ml PRP was injected into the left knee and 0.3 ml saline into the right knee in three groups at 4 weeks after the first collagenase injection. All rabbits were euthanized by CO inhalation at 4 weeks after saline and PRP injections.
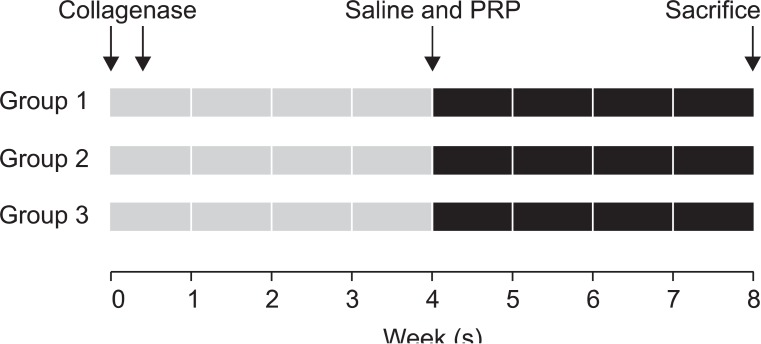
Fig. 3
Gross morphological (rectangular areas) and histological (hematoxylin and eosin staining, ×40) findings of knee osteoarthritis in group 1, 2, and 3 with intra-articular injection of saline (A-F) and platelet rich plasma (G-L).
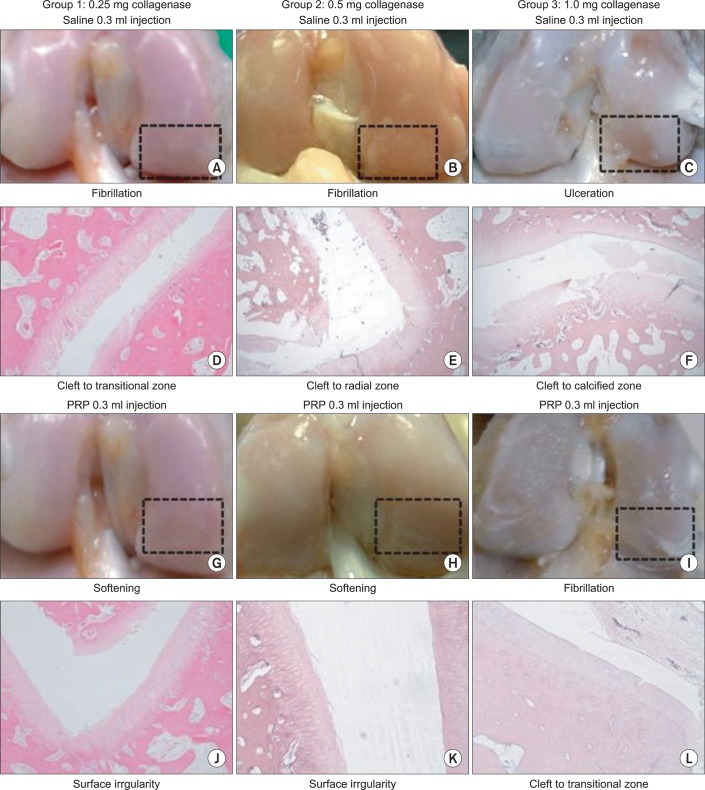
Fig. 4
Comparison of differences in gross morphological score between saline and PRP injected knees among three groups. YS: Yoshimi's score. *p<0.05 by Kruskall-Wallis test.
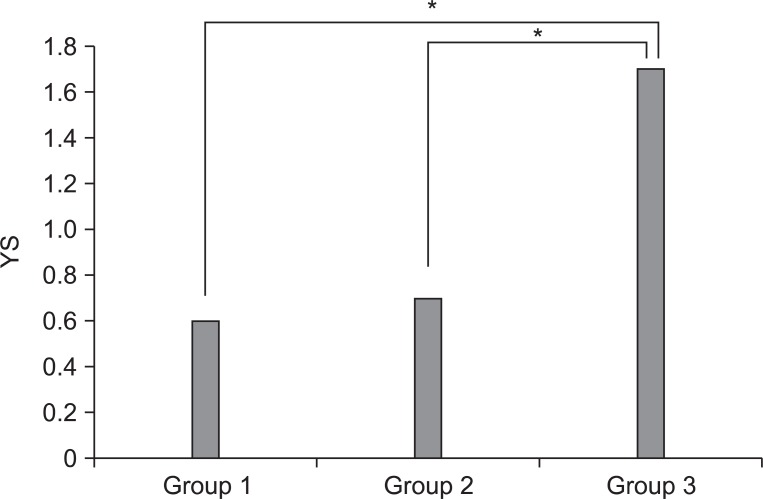
Fig. 5
Comparison of differences in histological evaluation score between saline and PRP Injected knees among three groups. MS: modified Mankin's score. *p<0.05 by Kruskall-Wallis test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download