INTRODUCTION
MATERIALS AND METHODS
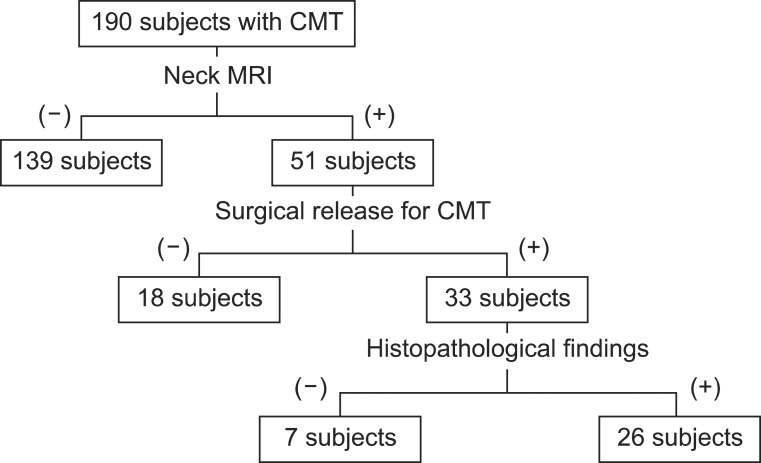
Enrollment of patients
Diagnosis of CMT
Magnetic resonance imaging
Surgical release for CMT
Review of the histopathologic findings
RESULTS
The presence of low signal intensity within the shortened SCM
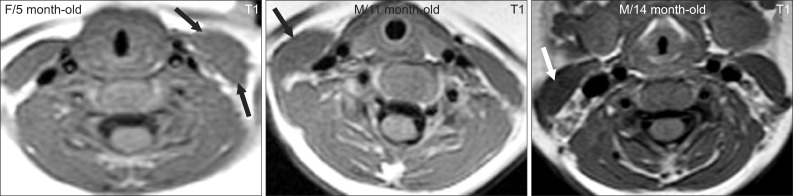 | Fig. 3Typical neck MRI findings of subjects who show a good response to stretching exercises. Thickened sternocleidomastoid muscles do not have low signal intensities (arrows). |
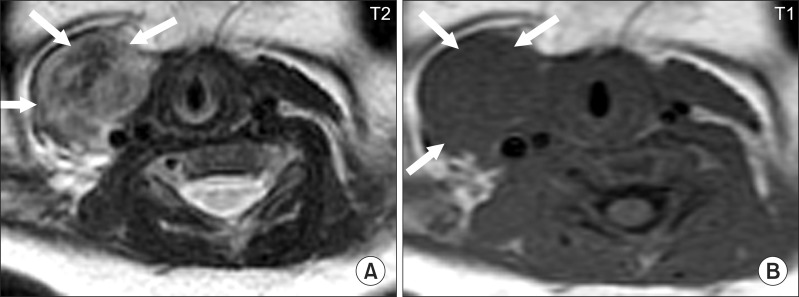
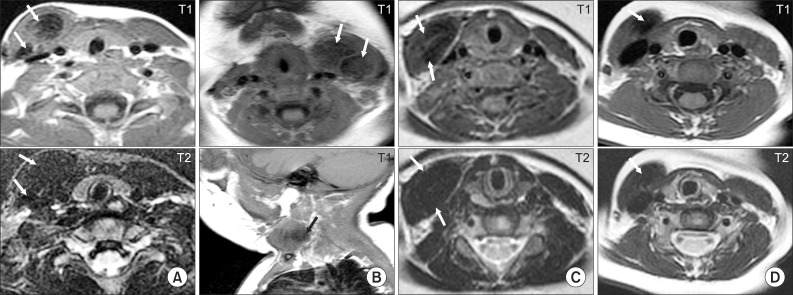 | Fig. 4Typical neck MRI findings of subjects who underwent surgical release for congenital muscular torticollis (CMT). (A) Eleven month old boy with right CMT showing low signal intensities on the T1- and T2-weighted axial images of both the sternal head and clavicular head (arrows) of the right sternocleidomastoid muscle (SCM). (B) Four month old girl with left CMT showing low signal intensities on the T1-weighted axial and sagittal images of the left SCM (arrows). (C) Six month old girl with right CMT showing low signal intensities on both the T1- and T2-weighted axial images of the right SCM (arrows). (D) Eighteen month old girl with right CMT showing low signal intensity on both the T1- and T2-weighted axial images of the right SCM (arrows). |
Identification of head(s) involved
Measurement of the thickness of the shortened SCM
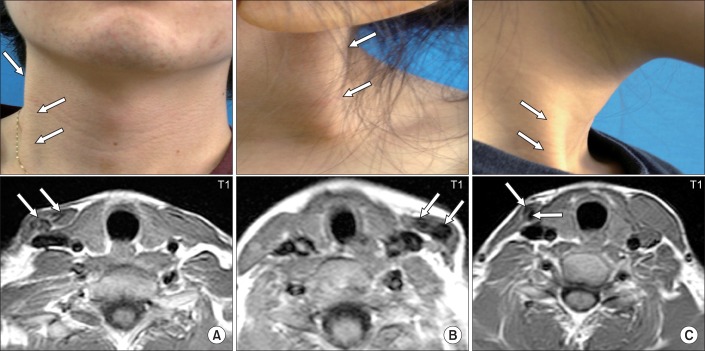 | Fig. 5Pictures showing subjects with atrophy or same thickness of the shortened sternocleidomastoid muscle (SCM) with low signal intensities on the T1-weighted axial images of the SCM. (A) Twenty-two year old man with right congenital muscular torticollis (CMT) showing a cord-like right SCM (arrows). Neck MRI showed low signal intensities (arrows) and did not show significant difference of thickness between the right and left SCM. (B) Five year old girl with left CMT showing a cord-like left SCM (arrows) and low signal intensity (arrows) on left SCM. (C) Twenty year old woman with right CMT showing atrophied cord-like right SCM (arrows) with low signal intensity (arrows) on right SCM. |
Review of the histopathologic findings
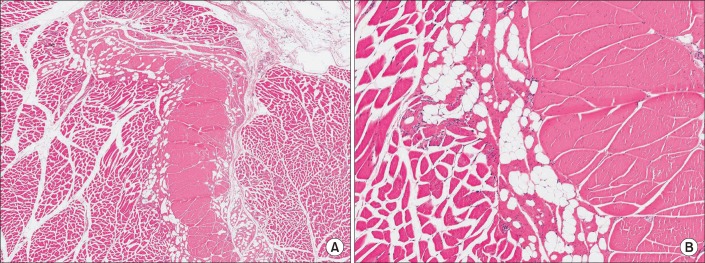 | Fig. 6The histologic findings of the normal sternocleidomastoid muscle without congenital muscular torticollis (H&E, (A) ×100; (B) ×200). |
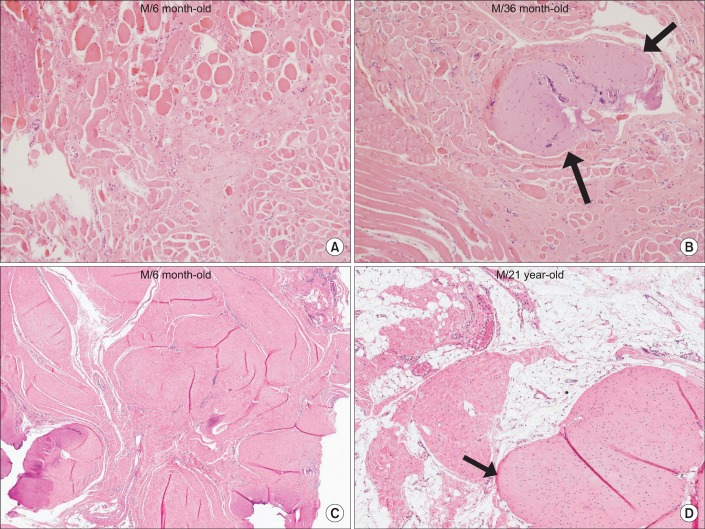 | Fig. 7The histopathological findings of the sternocleidomastoid muscle with congenital muscular torticollis. (A) Diffuse interstitial fibrosis with accompanying atrophic muscle fibers is noted (H&E, ×200). (B) Interstitial fibrosis with presence of aberrant tendon-like excessive dense connective tissue (arrows) (H&E, ×200). (C) Interstitial fibrosis with presence of aberrant tendon-like excessive dense connective tissue which was well-arranged (H&E, ×40). (D) Aberrant tendon-like excessive dense connective tissue and prominent fat infiltration (arrow) (H&E, ×40). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download