Abstract
Spinal cord infarction, especially anterior spinal artery syndrome, is a relatively rare disease. We report a case of spinal cord infarction caused by thoracoabdominal aortic aneurysm with intraluminal thrombus. A 52-year-old man presented with sudden onset paraplegia. At first, he was diagnosed with cervical myelopathy due to a C6-7 herniated intervertebral disc, and had an operation for C6-7 discetomy and anterior interbody fusion. Approximately 1 month after the operation, he was transferred to the department of rehabilitation in our hospital. Thoracoabdominal aortic aneurysm with intraluminal thrombus was found incidentally on an enhanced computed tomography scan, and high signal intensities were detected at the anterior horns of gray matter from the T8 to cauda equina level on T2-weighted magnetic resonance imaging. There was no evidence of aortic rupture, dissection, or complete occlusion of the aorta. We diagnosed his case as a spinal cord infarction caused by thoracoabdominal aortic aneurysm with intraluminal thrombus.
Because the spinal arteries tend not to be susceptible to atherosclerosis and emboli rarely lodge there, spinal cord infarction is a relatively rare disease. Spinal cord infarction occurs usually in the area of the anterior spinal artery, and it has been reported that atherosclerosis and thrombotic occlusion are quite uncommon in the anterior spinal artery. It is known that infarction in this area occurs secondary to disease of the extravertebral collateral artery or the aorta. Spinal cord infarction has been reported in cases involving cocaine users, cardiac and aortic surgery, aortic angiography, arteriovenous malformation, polyarteritis nodosa, and systemic hypotension.1 In South Korea, studies have reported that spinal cord infarction occurred due to aortic pathologies such as infrarenal aortic surgery, complete aortic occlusion, aortic dissection, and aortic intramural hematoma.2-4 However, no study has reported a case where spinal cord infarction spontaneously occurred due to asymptomatic aortic aneurysm with intraluminal thrombus without involving dissection or rupture. We experienced a case where spinal cord infarction spontaneously occurred due to asymptomatic aortic aneurysm with intraluminal thrombus, and herein report the case together with a discussion of the relevant literature.
A 52-year-old male patient presented to the emergency room of a local hospital due to sudden weakness in both lower limbs. The patient was taking medications for hypertension and had a 60 pack-year smoking history, but reported that he had no history of diabetes, hyperlipidemia or cardiac disease. He described that he had an electrifying feeling in the dorsal spine at dinner and then 5 minutes later, he experienced weakness in both lower limbs. According to his medical record presented to the hospital, he had no abnormalities of consciousness and cognition, and no manifestations such as fever suggesting systemic infection or inflammation. The muscle strength using the Medical Research Council Scale was grade 5/5 in both upper limbs and 0/0 in both lower limbs, and numbness was observed below the T7 dermatome. Magnetic resonance imaging (MRI) (sagittal image of whole spine and axial image of cervical spine) and cervical computed tomography (CT) which were performed 3 hours after the onset of symptoms showed that the C6-C7 intervertebral disc was compressing the cervical spinal cord on the right central side (Fig. 1). Discectomy of the C6-C7 intervertebral disc and anterior interbody fusion of the C6-C7 vertebrae were performed as emergency care. The patient was transferred to the Department of Rehabilitation in our hospital for active rehabilitation treatment 1 month after the onset of the symptoms, with no clear postoperative functional improvement in the lower limbs. At the time of transfer to our hospital, muscle strength grade was 1/1 for hip flexion, 1/1 for knee extension, 1/2 for ankle dorsiflexion, 2/2 for ankle plantar flexion, and 1/1 for extensor hallucis longus muscle without spasticity. Sensory tests showed that pain and temperature senses were decreased, although touch, vibration, and position senses were normal in the T8-T12 dermatomes, and all senses were normal in the other dermatomes including the anal region. Deep tendon reflex was normal in both upper limbs but was absent in both lower limbs. Babinski's sign and ankle clonus were absent. The anal sphincter was relaxed and voluntary anal contraction could not be implemented. Placement of a Foley catheter inserted through the urethra before the surgery was maintained for voiding. The patient's neurologic level of injury was T7 and the American Spinal Injury Association Impairment Scale was Grade C. This was inconsistent with the general symptoms of cervical myelopathy caused by C6-7 herniated intervertebral disc.
On the day of transfer to our hospital, the patient had a fever and an abdominal CT was performed to identify the cause of the fever. The abdominal CT showed prostatitis and a thoracoabdominal descending aortic aneurysm of 4.1 cm in diameter. Approximately 50% of the inner diameter of the aneurysm was found to be filled with thrombus (Fig. 2). The results of electrocardiography and immunology tests including a venereal disease research laboratory test were normal. Blood lipid tests showed that total cholesterol and low density lipoprotein (LDL) levels were normal (149 mg/dl and 91 mg/dl, respectively), high density lipoprotein (HDL) level decreased to 22 mg/dl, and triglyceride (TG) level increased to 186 mg/dl. Antibiotics were administered for prostatitis, which was suspected as a cause of the fever, and a suprapubic cystostomy was performed. In addition, after consultation with the department of internal medicine, aspirin and atorvastatin calcium were administered for thrombus of thoracoabdominal aortic aneurysm and dyslipidemia.
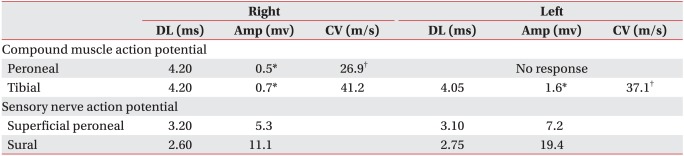
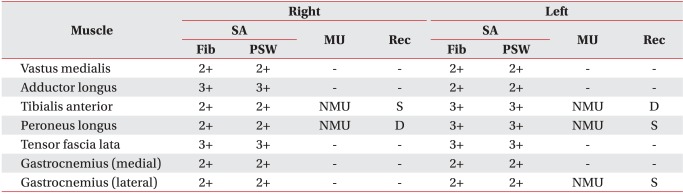
An electrophysiologic study was performed 5 weeks after the onset of the symptoms. Although the findings of a sensory nerve conduction study on both superficial peroneal and sural nerves were within normal ranges, the findings of a motor nerve conduction study on both peroneal and posterior tibial nerves showed that the amplitude of compound muscle action potential was either decreased or no compound muscle action potential was elicited (Table 1). Needle electromyography showed abnormal spontaneous activities in all the myotomes from the L2 to S1 on both sides. Also, no motor unit action potential was observed, or recruitment was found to have decreased (Table 2). The results of a somatosensory evoked potential test on the median and posterior tibial nerves were normal on both sides. Based on clinical findings from the electrophysiologic study, thoracolumbar MRI was performed to detect any thoracolumbar lesion that could indicate the impairment of lower motor neurons. The sagittal T2-weighted image showed linear high signal intensity in the spinal cord from the T8 to the cauda equine level, and an axial T2-weighted image revealed 'snake eyes' appearance5 where the anterior horns of gray matter of spinal cord had high signal intensity (Fig. 3).
Based on the patient's medical history, clinical symptoms, abdominal CT and thoracolumbar MRI findings, we considered that the cause of acute both lower limbs' paralysis was spinal cord infarction caused by thoracoabdominal aortic aneurysm with intraluminal thrombus. We decided to maintain the current drug and rehabilitation treatments as the spinal cord infarction had exceeded acute phase. At present, muscle strength in the patient's lower limbs has not markedly improved compared with that at his presentation to our hospital. The Foley catheter placed in suprapubic vesical fistula has been removed and the patient now voids by Crede maneuver.
The patient experienced transient electrifying pain in the back, and 5 minutes later rapid flaccid paralysis of both lower limbs, sensory dissociation of T8-T12 dermatomes, and sphincteric dysfunction. These symptoms correspond with the manifestation of anterior spinal artery syndrome. The segmental artery emerging from the aorta branches out into the anterior radicular artery. The anterior radicular artery forms the anterior spinal artery, supplying blood flow to the anterior 2/3 of the spinal cord. Infarction in the anterior spinal artery is called anterior spinal artery syndrome and causes flaccid paralysis below the level of the lesion.1 Transient lancinating or dull pain in the back may precede the manifestation of symptoms that rapidly develop in a few minutes or hours, and are accompanied by paralysis of sphincteric function. In addition, although pain and temperature senses are lost or decreased due to damage of the spinothalamic tract, the posterior columns are preserved, resulting in sensory dissociation where vibration and position senses are maintained.1,6
MRI is useful for the diagnosis of spinal cord infarction. Excluding the possibility of lesions that compress the spinal cord or possibility of lesions that exist within the spinal cord, a T2-weighted image can reveal the high signal intensity in the spinal cord infarction region.1,5 However, in the early stage of the infarction, a T2-weighted image may not show any change in the signal intensity.5 In this case, 2 months after the manifestation of symptoms, the sagittal T2-weighted MRI taken at our hospital showed linear high signal intensity in the thoracolumbar spinal cord, and axial T2-weighted MRI showed 'snake eyes' appearance where high signal intensity was observed in the anterior horns of gray matter of the spinal cord.5 These findings suggest spinal cord infarction, and are consistent with the fact that motor function loss was higher than sensory function loss in the patient. However, the sagittal T2-weighted MRI of whole spine taken 3 hours after the manifestation of symptoms at the previous hospital did not show change in signal intensity in the thoracolumbar spinal cord. In contrast, the finding that herniation of the C6-C7 intervertebral disc compressed the spinal cord may have led to the diagnosis of cervical myelopathy caused by the C6-C7 herniated intervertebral disc. A case of acute lower limb paralysis due to non-traumatic herniation of cervical intervertebral disc, though rare, was reported in another study. In such a case, prompt decompression surgery is required to prevent neurological impairment from progressing to irreversible impairment.7 In this case, it is believed that the emergency surgery was performed because of consideration that prompt decompression surgery was required. However, given that the patient's symptoms were consistent with manifestations of typical spinal cord infarction, and MRI cannot show abnormal findings in the early phase of spinal cord infarction, it is believed that follow-up thoracolumbar MRI was required even after the surgery.
In several studies, aortic pathologies that cause spinal cord infarction included cardiac or aortic surgery, rupture of aortic aneurysm, aortic dissection, aortic intramural hematoma, and acute aortic occlusion.1-4,8 In this patient's case, however, no findings such as aortic dissection or rupture of an aortic aneurysm were observed and no surgery or procedure had been previously performed for aortic aneurysm. In addition, although the aneurysm was filled with thrombus, the degree of thrombus was not sufficient to cause aortic occlusion, and infarction of other organs including the lower limbs due to thrombus or embolus did not occur. In this patient's case, it is assumed that the spinal cord infarction possibly occurred due to the following mechanisms. Because a large T10 or L1 anterior radicular artery, better known as the artery of Adamkiewicz, supplies blood to the thoracolumbar spinal cord,1 detachment of a portion of thrombus of the aortic aneurysm might have caused embolism of the artery of Adamkiewicz. In addition, the artery of Adamkiewicz is known to usually originate from the left side,8 and in this patient's case, considering the accumulation of thrombus in the left side of the aorta, it was likely that the accumulation of thrombus blocked the origin of the segmental artery that branches out into the artery of Adamkiewicz.
Although we considered that this patient's case was due to thoracolumbar spinal cord infarction caused by thoracoabdominal aortic aneurysm with intraluminal thrombus, there are several limitations to make an accurate diagnosis. First, as cerebrospinal fluid was not examined at the time of manifestation of the symptoms, the possibilities of a demyelinating, infectious, or inflammatory lesion of spinal cord cannot be excluded. However, given that the patient's neurological symptoms rapidly progressed within several minutes, there were no findings suggestive of systemic infection or inflammation such as fever at the time of manifestation of the symptoms, no viral diseases existed before the manifestation of the symptoms, and the lesions existed in the anterior horns of gray matter instead of lateral and posterior funiculi, it is believed that this case was more likely due to spinal cord infarction.9 Another limitation is that the relation between thrombus of aortic aneurysm and impairment of blood flow of the spinal cord could not be objectively ascertained because angiography could not be performed at the time of manifestation of the symptoms. Despite this, based on the other studies that reported numerous aortic lesions as the cause of spinal cord infarction, and a case in a foreign country where thrombus of aortic aneurysm spontaneously caused spinal cord infarction,10 we believed that the cause of spinal cord infarction was thoracoabdominal aortic aneurysm with intraluminal thrombus.
When acute lower limb paralysis occurs without external injury, the possibility of spinal cord infarction together with other etiologies should be considered, and elaborate neurological examination and MRI can be useful to differentiate them. In addition, aortic pathologies as a possible etiology should be examined, and it should also be considered that spinal cord infarction may occur even by asymptomatic aortic aneurysm with intraluminal thrombus, without rupture or dissection.
References
1. Roppr AH, Samuels MA. Adams and Victor's principles of neurology. 2009. 9th ed. New York: McGraw-Hill Professional;p. 1202–1205.
2. Kim HT, Cho WH, Kim HC. Spinal cord ischemia related to infrarenal aortic pathology and surgical procedure. J Korean Soc Vasc Surg. 1999; 15:88–93.
3. Oh EJ, Jeong SW, Park JK, Hong KS. Painless aortic dissection simulating Guillain-Barre syndrome. J Korean Soc Clin Neurophysiol. 2005; 7:49–51.
4. Han HS, Shin DS, Lee SH, Park SW, Park HY, Chang H, Kim YS, Cho KH, Jang SJ. A case of spinal cord ischemia induced by aortic intramural hematoma. Korean J Stroke. 2004; 6:121–123.
5. Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE. Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology. 2002; 44:851–857. PMID: 12389137.

6. Sliwa JA, Maclean IC. Ischemic myelopathy: a review of spinal vasculature and related clinical syndromes. Arch Phys Med Rehabil. 1992; 73:365–372. PMID: 1554311.

7. Liu C, Huang Y, Cai HX, Fan SW. Nontraumatic acute paraplegia associated with cervical disk herniation. J Spinal Cord Med. 2010; 33:420–424. PMID: 21061902.

8. Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996; 47:321–330. PMID: 8757000.
9. Jacob A, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Semin Neurol. 2008; 28:105–120. PMID: 18256991.

10. Fairhead JF, Phillips D, Handa A. Embolic spinal cord infarction as a presentation of abdominal aortic aneurysm. J R Soc Med. 2005; 98:59–60. PMID: 15684355.

Fig. 1
Magnetic resonance imaging of cervical spine. Sagittal T2-weighted image reveals degeneration and diffuse bulging of disc (arrow) at the C6-7 level (A). Axial T2-weighted image shows a right-side central focal protruded disc (arrow) at the C6-7 level (B).
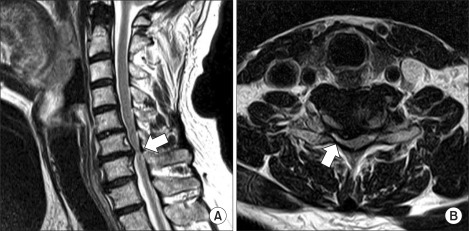
Fig. 2
Enhanced computed tomography scan shows aneurysmal dilatation with intraluminal thrombus (arrow) at the T9 (A) and L1 (B) level.
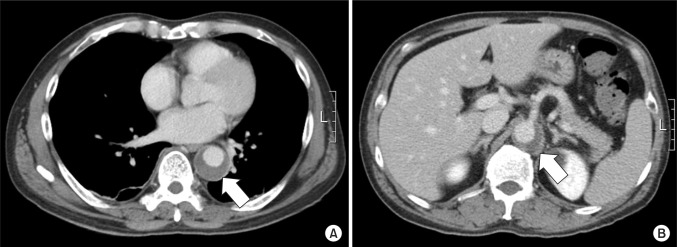
Fig. 3
Magnetic resonance imaging of thoracolumbar spine. Sagittal T2-weighted image reveals increased intramedullary signal intensity (arrow) from the T8 to cauda equina level (A). Axial T2-weighted image at the T11 level shows bilateral hyperintensities corresponding to the anterior horns of gray matter ('snake eyes' appearance) (arrow) (B).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download