Abstract
Objective
To correlate existing evaluation tools with clinical information on Duchenne muscular dystrophy (DMD) patients following age and to investigate genetic mutation and its relationship with clinical function.
Method
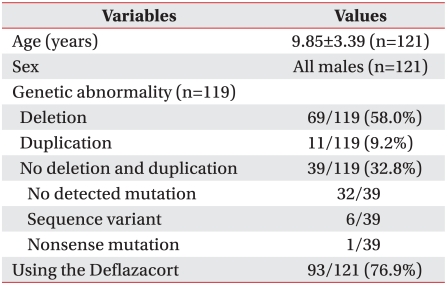
The medical records of 121 children with DMD who had visited the pediatric rehabilitation clinic from 2006 to 2009 were reviewed. The mean patient age was 9.9±3.4 years and all subjects were male. Collected data included Brooke scale, Vignos scale, bilateral shoulder abductor and knee extensor muscles power, passive range of motion (PROM) of ankle dorsi-flexion, angle of scoliosis, peak cough flow (PCF), fractional shortening (FS), genetic abnormalities, and use of steroid.
Results
The Brooke and Vignos scales were linearly increased with age (Brooke (y1), Vignos (y2), age (x), y1=0.345x-1.221, RBrooke2=0.435, y2=0.813x-3.079, RVignos2=0.558, p<0.001). In relation to the PROM of ankle dorsi-flexion, there was a linear decrease in both ankles (right and left R2=0.364, 0.372, p<0.001). Muscle power, Cobb angle, PCF, and FS showed diversity in their degrees, irrespective of age. The genetic test for dystrophin identified exon deletions in 58.0% (69/119), duplications in 9.2% (11/119), and no deletions or duplications in 32.8% (39/119). Statistically, the genetic abnormalities and use of steroid were not definitely associated with functional scale.
Muscular dystrophy (MD) is a devastating inherited neuromuscular disease characterized by early onset and progressive course. Duchenne muscular dystrophy (DMD) is the most common subtype of MD with an incidence of 1 in 3,500 to 6,000 live male births.1 The DMD is a pattern of X-linked recessive inheritance characterized by lack of dystrophin, which provides structural stability to the dystroglycan complex, located on the cell membrane within muscle tissues.2,3 As a result, progressive proximal muscle weakness usually appears in male children before age 5 years, and eventual gait difficulty follows. In general, DMD patients increasingly rely on a wheelchair by age 15 years, and death usually occurs before or at 20 years of age.4-6 As the disease progresses, most patients present various symptoms including joint contractures and muscle weakness in bilateral limbs, scoliosis, aggravated lumbar lordosis,7 dilated cardiomyopathy,8,9 and dyspnea due to respiratory muscle failure.10,11 There are different kinds of treatment options depending on the goal of treatment; steroids, such as Deflazacort for walking and cardiac function,12,13 bracing for deformities of joints and spine, and physical or aqua therapies for weakness or poor general condition.14 However, the aforementioned treatments only slow down the disease progression rather than provide a complete cure. At present, the life expectancy of DMD patients have been extended by the development of medical technology, an increase in rehabilitation facilities, and an increased accessibility of hospital for general medical care.15 In addition, molecular and genetic therapies, such as exon skipping, mutation-specific treatment using nonsense codon suppressors, and gene therapy using viral and nonviral vectors, have been tried. Effects of these treatments are discussed, and expected to be used for the treatment of DMD patients in near future.16-18
There are a few objective functional scales for DMD patients, but these are not commonly used. Brooke scale is an evaluation tool for upper extremity function with limitations for estimating lower extremity function and joint contractures.19 Vignos scale is a popular evaluation index primarily for lower extremity function, however, the grading is not clear.19 In addition, according to an increased power wheelchair use led to reduced demand for orthosis, such as knee-ankle-foot orthosis (KAFO), subscales of the Vignos scale need to be revised. In 2006, Lue YJ et al. reported on the development of a muscular dystrophy-specific functional rating scale (MDFRS),20 which is a comprehensive evaluation tool that takes into account both upper and lower extremities function and contractures in MD. However, respiratory and cardiac functions are difficult to check and have rarely been used in the clinical setting.
Despite increased life expectancy and new developing treatment techniques, comprehensive and clinical evaluation tools are still lacking. Moreover whether the existing assessment tools accurately reflect the functioning of DMD patients has not reported in detail. In this study, the objective was to correlate clinical information to already existing assessment tools and age. Furthermore, we investigated the genetic mutation in the dystrophin gene and its relationship with function, as well as functional change following Deflazacort use.
The medical records of DMD patients between January 2006 and February 2009 at the pediatric rehabilitation clinic were reviewed retrospectively. All 121 subjects were male and had a mean age of 9.85±3.39 years at their first visit (Table 1). The diagnosis of DMD was confirmed by genetic testing, muscle biopsy, and clinical findings by pediatrician and rehabilitation medicine specialist or previous diagnosis in another hospital. Evaluation exclusion criteria including Brooke scale, Vignos scale, and muscle power were; wearing splint or orthosis due to recent trauma or operation, medical problems (pneumonia that could affect the general condition), and a recently deteriorating condition due to seizures. Patients were also excluded from the study if records related to evaluation were incomplete because of poor cooperation.
Items reflecting function and physical condition of DMD patients were selected from the medical records based on previous studies. The selected items include Brooke scale, Vignos scale, passive range of motion (PROM, °) of bilateral ankle dorsi-flexion,21-23 muscles power (N) of bilateral shoulder abductors (deltoid muscles) and knee extensors (quadriceps muscles),21,24,25 Cobb angle (°) to confirm scoliosis,26-28 peak cough flow (PCF, L/min) for respiratory function,29,30 and fractional shortening (FS, %) for left ventricle function.31 All items were not completely documented for each patient. Records of 121 patients at initial visit, as well as follow up sessions (maximum of 6 sessions) were reviewed. Each item was measured by a physiatrist with the following standards. Brooke and Vignos scales were checked at every visits in outpatient clinic (Table 2). The PROM of bilateral ankle dorsi-flexion was measured in sitting or supine position with knees flexed to 90 degree by a goniometer with one axis parallel placed at the fibular shaft and the others at the fifth metatarsal bone. Passive dorsi-flexion range of the ankle was recorded as positive, neutral was recorded as 0, and plantar flexion contracture of ankle that limits dorsi-flexion was recorded as negative. Muscle power was assessed by hand held dynamometer FGN-50 (Nidec-Shimpo corporation, Kyoto, Japan) at bilateral shoulder abductors and knee extensors referred to reported method by Palmieri et al.25 The shoulder abductor muscle power was examined from the lateral forearm when sitting upright, with the shoulder abducted to a 45 degree angle with the elbow was straight and with the forearm in a pronated position. The knee extensor muscle power was examined from the anterior surface of distal shaft just proximal to ankle joint in the sitting position with a 90 degree flexed position.19 Cobb angle was the method of measuring kyphosis, and was defined as the angle between a line drawn parallel to superior endplate of the superior end vertebra and a second line drawn along the inferior end plate of the inferior end vertebra by Antero-Posterior view of whole spine on X-ray. The PCF was measured by flowmeter EN 13826 (Micro Medical, Basingstoke, UK) in a wheelchair or in the chair sitting position. Most patients' PCF were tested more than twice, and values considered to be significant by the examiner were recorded. If PCF was tested only once, the result was recorded when checked value was judged as meaningful by the examiner. The FS, [(left ventricle end-diastolic diameter)-left ventricle end-systolic diameter)×100]/(left end-diastolic diameter), which reflect left ventricular function was measured by pediatric echocardiography.32
In addition to the above items, previous genetic test results were gathered according to type of genetic mutation, including exon deletion and duplication, no deletion and duplication, and the methods. There were 119 available genetic results. Genetic test methods used were multiplex polymerase chain reaction (mPCR, 26 sites: pb, pm, exons 3, 4, 6, 8, 12, 13, 16, 17, 19, 32, 34, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 60)33 until July 2005, and multiplex ligation-dependent probe amplification (MLPA)34 since August 2005. Studies comparing exon deletion number with functional status have not been reported until now. Assuming an increased number of exons deletion, decrease functional status, and differences in the clinical items evaluated according to exon deletion number. Brooke scale, Vignos scale, and PROM of bilateral ankle dorsi-flexion were compared between single deletion and four or more exon deletion among the subjects with a highly frequent deletion site between exon 44 and 55. Ninety-three of the 121 patients were treated with Deflazacort and 28 were not, and these data underwent analysis. The impact of Deflazacort, a type of steroid, which might have affected patient function, was confirmed using the Vignos scale to verify the risk of its effect on the patients' function in those who used it.
The correlation of each item with age was analyzed by linear regression model, and R square value (R2) was confirmed by depicting trend line. Due to some missing data, a mixed model was applied to statistically analyze the data including functional status at specific age, clinical physical condition, and a serial functional change. In the genetic tests, difference of function depending on number of exon deletion (single versus 4 and more exons deletions) was compared with Brooke scale, Vignos scale, and PROM of bilateral ankle dorsi-flexion considering the age at the time of measurement. Mann-Whitney U-test, a nonparametric method, was used due to small number of subjects and without equal variance. Changes in Vignos scale according to used of Deflazacort were identified by regression analysis at Medical Research Collaborating Center (MRCC). The value of significance was set at p<0.05 and 95% confidence intervals (CI) were provided.
The higher the score, the more severe the decrement in function on the Brooke and Vignos scales. In the Brooke scale, total 252 scores in 90 of 121 patients were linearly increased according to increasing age, and R2 of trend line was 0.435 (Brooke scale [y1], age [x], y1=0.345x-1.221, p<0.001) (Fig. 1-A). In Vignos scale, total 250 scores in 89 of 121 patients were also linearly increased according to increasing age, and R2 of trend line was 0.558 (Vignos scale [y2], age [x], y2=0.813x-3.079, p<0.001) (Fig. 1-B) which demonstrates lower extremity function was correlated relatively with age. Bilateral ankle dorsi-flexion angles showed a decreasing tendency as age increased. R square value for right was 0.364 (n=218, p<0.001, right ankle dorsi-flexion PROM (y1), age (x), y1=-4.535x+35.362) and R2 for left was 0.372 (n=219, p<0.001, left ankle dorsi-flexion PROM (y2), age (x), y2=-4.525x+35.568) (Fig. 2). In other words, as age increased, the more the ankle plantar contracture progressed in DMD.
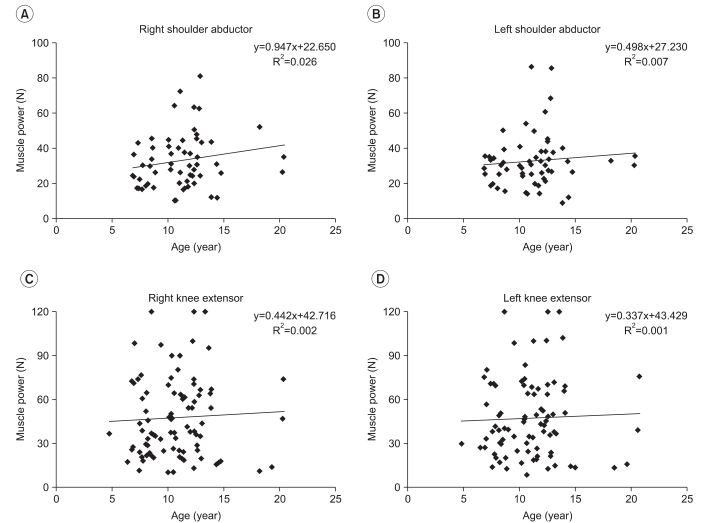
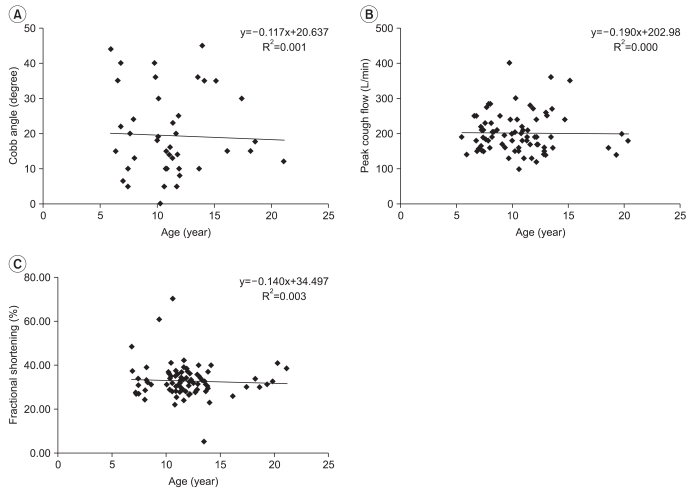
R square value was very low for bilateral shoulder abductor/knee extensor muscles power showing 0.026 (n=59, p=0.219)/0.002 (n=87, p=0.659) on the right and 0.007 (n=57, p=0.523)/0.001 (n=86, p=0.735) on the left (Fig. 3). There was no correlation between age and clinical indexes (Fig. 4), which included 43 cases of Cobb angle (n=40, R2=0.001, p=0.828), 80 cases of PCF (n=59, R2<0.001, p=0.926), and 84 cases of FS (n=56, R2=0.003, p=0.624).
We reviewed 119 genetic results from 121 patients; records of genetic results were not present for 2 patients. The genetic mutation in 71 subjects (59.7%) was confirmed by MLPA, 22 (18.5%) by mPCR, and 16 (13.4%) by mPCR combined with MLPA. A specific method of genetic test was not recorded in 10 subjects; however, results of genetic mutation were recorded. Genetic abnormality was categorized by exon mutation, such as exon deletion of 67 (58%), duplication of 11 (9.2%), and no deletion or duplication of 39 (32.8%, Table 1). Among 39 patients without deletion or duplication, sequence variant was verified at 6 and one patient had a nonsense mutation.
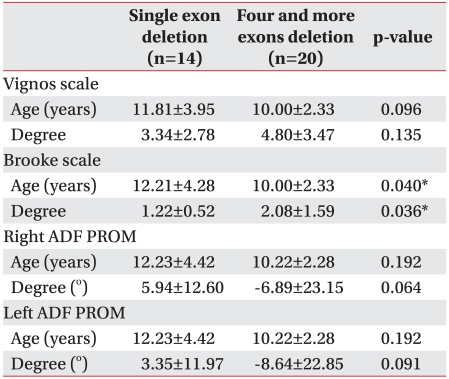
In the 69 patients with deletions, the majority (30 patients) had a deletion of exon 50, other deletions included; 29 patients without exon 47, 28 patients without exon 46, and 28 patients without exon 49. It is well known that reported high frequently deletion sites are exon 1 to 20 and 44 to 55; exon deletions in 8 patients (11.6%) were located within exon 1 to 20, 52 patients (75.4%) were located within exon 44 to 55, and 9 patients were situated at variable sites. The number of single exon deletions was 6 subjects at exon 45, 4 at exon 51, 2 at exons 44 and 50, and 1 at exons 12, 21 and 57. On classifying the patients according to the number of deficits between 44 and 55, the most common deletion site, a single exon deletion was found in 14 patients, 2 exons deletion in 7 patients, 3 exons deletion in 10 patients, 4 exons deletion in 3 patients, and more than 5 exons deletion in 17 patients. The functional scales and gene deletions (single versus 4 and more exons deletions) were compared, because the 4 exons deletion was the median value among the total number of deletion excluding single deletion base on number of patients. For patients with a single deletion, the mean Vignos scale score was 3.34±2.78 (12 subjects/22 cases, mean age 11.81±3.95 years), the mean Brooke scale score was 1.22±0.52 (12 subjects/23 cases, mean age 12.21±4.28 years), and the right ankle dorsi-flexion PROM was 5.94±12.60° and left was 3.35±11.97° (9 subjects/17 cases, mean age 12.23±4.42 years). In patients with 4 and more deletions, the Vignos scale score was 4.80±3.47 and the Brooke score was 2.08±1.59 (15 subjects/32 cases, mean age 10.00±2.33 years), the right ankle dorsi-flexion PROM was -6.89±23.15° and the left was -8.64±22.85° (13 subjects/28 cases, mean age 10.22±2.28 years, Table 3). On the Vignos scale, the difference of age between the 2 groups was not significant (p=0.096) and difference in mean score, which was approximately scale 1, was not significant also (p=0.135). The difference in Brooke scale was statistically significant between the 2 groups (p=0.032), however, upper extremity function in most patients was good (Brooke scale 1 or 2) for each group and the mean age of single exon deletion was higher than 4 or more deleted exons (p=0.04). Because of these biases, the clinical significance was unclear. Bilateral ankle dorsi-flexion PROM, mean scores of Brooke scale were not different between the 2 groups on the left (p=0.064) and right (p=0.091), and mean age at time of exam (p=0.192).
Ninety-three (76.9%) patients of 121 used Deflazacort (Table 1). The effects of using Deflazacort were analyzed to change the Vignos scale, which was correlated with disease progression in the previous results. Contrary to previous research, patients with Deflazacort were at increased risk of an increasing Vignos scale compared with patients without Deflazacort. This result indirectly suggests that Vignos scale was not affected by the use of Deflazacort or was increased by Deflazacort, however, this was not statistically significant (p=0.59).
The strength of the study is that it had lots of data collected from many DMD patients and confined subjects to DMD patients instead of covering various kinds of MD, which made homogeneity enhanced. In addition, the study made a comprehensive evaluation of cardiopulmonary function by making good use of the Vignos scale, the Brooke scale, PCF, and FS. In addition, this study is significant in that it objectively examined the relationship between the change in patient function and age by the use of evaluation tools and lots of genetic information.
The limitation of this study is that there is a great deal of missing data and it did not examine the change in patient functions by age, progressively. If there had been no missing data, we could have obtained a result by analyzing values measured according to change in age through Analysis of variance (ANOVA). However, this study could not examine the change in function, as there was missing data and age periods for evaluating function were different. In addition, the study used partial aspects of materials due to difficulty of grasping general condition of patients as the study was dependent upon retrospective study, which was dependent upon medical records. Besides, the study did not clearly distinguish BMD caused by in-frame deletion as genetic test of dystrophin gene conducted by our clinic did not check all sequences and the methods of gene diagnosis were not identical to each other.
One of the purposes of this study was to examine whether evaluation tools showed a tendency properly that the functions weaken as the disease progresses and the age increases when functional evaluation tools are actually applied to DMD patients. To this end, the work on choosing items that can reflect the function and clinical status of DMD patients was conducted. Functional evaluation tools and clinical assessment of DMD patients reported by previous research were examined through expert meeting. A total 7 items, including cardiac assessment by adding significant items that were considered necessary and can be easily checked in outpatient clinics were determined. Gowers' sign, in which a patient pushes himself up from his knee with his hand when he stands up after laying face down or sitting on the ground, was considered positive. In cases in which mental retardation (MR)35 was confirmed and the case in which MR was not confirmed under Korean Educational Development Institute-Wechsler Intelligence Scale for Children (KEDI-WISC), and Korean-Child Behavior Checklist (K-CBCL) were distinguished by requesting the test child psychiatrist. The relation between Gowers' sign and deteriorating intelligence was not analyzed due to missing data and the difficulty in comparing according to change in age. Among the total 121 DMD patients, those who showed a positive Gowers' sign were 24, those who showed negative reaction in Gowers' sign were 9; and those who did not show clear reaction were 3 and those who did not have data were 85. There were 17 patients with MR; patients who did not show MR were 48 and 56 patients did not have data.
It was found that among tools that evaluate DMD patients, Vignos scale showed the pattern that increases linearly according to the change in age most significantly. This finding means that the reduction in function appears linear in proportion to the increase in age and it is expected that Vignos scale will help predict the change in functions of DMD patients. It was found that the Brooke scale increased linearly in proportion to the increase in age, whereas PROM of bilateral ankle dorsi-flexion decreased. It is expected that these findings are useful in inferring the change in functions by age.
It is reported that dilated cardiomyopathy is common in DMD patients, in particular those DMD patients who have difficulty in walking due to severe DMD.10 However, this study found that FS did not decrease in proportion to an increase in age. This result may be attributed to the fact that most of the subjects in this study were in relatively good condition, which prevented the deterioration in heart function from clearly being shown. As individual difference is large in FS, a significant result can be obtained when the change in patients is analyzed progressively; however, because the result of echocardiography used in this study was obtained by comparing the results at a certain point of subjects which prevented the deterioration of function being identified. It is estimated that the fact that this study did not evaluate the functioning of DMD patients by using the history of taking heart disease related medicines and B-type natriuretic peptide (BNP),32 which is commonly used as an indicator of heart function and limited the evaluation of heart function. It was also found that bilateral shoulder abductor and knee extensor muscles power, Cobb angle and PCF did not show a linear increase or a linear decrease in proportion to age. It is estimated that the result may be attributed to the fact that muscular strength of DMD patients increased in the early stage, but decreases as DMD progresses and scoliosis develops in only some parts and worsens sharply in proportion to age.
Dystrophin gene is located at Xp21.2 and large gene consisting of 79 exons with the size of 2.4 Mb. Deletion is most common in the dystrophin gene mutation and the generation of duplication was found in 5-10% of the patients36 and variation, such as point mutations, microdeletions and microinsertions were found in 20-30% of the patients.37 Exon deletion, which is most common in dystrophin gene mutation, was found in 58.0% (69/119) of the patients with similar findings in the results of previous research (55-65%). It was found that duplication was 9.2% (11/119), which coincides with result of previous studies.36,38 The site of gene deletion generation is called a 'hot spot' and the hot spot is the first 20 exons located within 500 kb from the end of 5' and is located between exon 44 and exon 55 located within 1,200 kb from first exon.39 It is reported that the deletion frequency is highest in the middle part of 1,200 kb.33,36 This study revealed that greatest dystrophin gene deletion was found exon 50 that is located between exon 44 and 55 and exon 46, exon 47, and exon 49 was highly frequent deletion site.
Like DMD, BMD which is somewhat slight in symptoms can be generated by dystrophin gene deletion. If the deletion site moves the translational reading frame of mRNA, transcription ends in early stage in "out of frame deletion" and dystrophin protein is not formed and severe DMD is revealed. On the contrary, despite deletion, if the translational reading frame is preserved, abnormal protein or protein with small molecular mass generates in "in frame deletion" and slight BMD becomes revealed.40 Two tests used by our hospital are known as the method that can accurately identify deletion and duplication in the dystrophin gene, but as mPCR selectively examines those exons in which deletion is common, mPCR is not enough to accurately identify gene deletion or duplication. Thus, our hospital reexamines patients by using MLPA though medical checkup and was done through mPCR in this case; whether there is deletion or duplication that is revealed through MLPA is examined. Statistical analysis was conducted through gene diagnosis after checking whether there was something remarkable in the results reported by the laboratory medicine department of our hospital.33,41 However, in MLPA implemented, the total genetic sequencing of dystrophin was not done and therefore it was impossible to gain information on in frame deletion and out of frame deletion.
This study examined whether there is difference by comparing the Vignos and Brooke scales, PROM of bilateral ankle dorsi-flexion of patients who had single deletion and 4 or more deletions among deletions shown between exon 44 and 55. Comparisons were made under the assumption that the more exons deletion in DMD patients, the worse the function in DMD patients but statistical significance was not found except Brooke scale. In the case that score was recorded and the number of subjects was small, Brooke scale shows good function (1 or 2 point) and the difference in age was not corrected, it was difficult to interpret that there was a difference in functioning. Further research is needed because functional scale showed a tendency of becoming deteriorated when there is 4 or more exon deletions. The tools that can evaluate DMD patients comprehensively and objectively are needed for this type of further research and if such tools are developed and used, the correlation functions evaluated in the clinical setting and the result of gene diagnosis can be fully explained.
The influence of steroids, which has been reported to delay natural progress of the disease, has on walking of DMD patients was examined,11,12 and the result of this study was different from the report mentioned above. This difference may be attributed to the errors that Deflazacort was used at the time when walking became not easy and drug therapy was given to the patients who showed deterioration of functions. This result is considered as limit that is shown in analysis which uses retrospective data and estimated as indication bias. Introducing a system that measures severity of the disease or introducing propensity scoring matching (PSM) that can evaluate the relation between various factors and medical treatments will help overcome indication bias.
The result of this study revealed that existing functional evaluation tools have limits in making a comprehensive evaluation of the functions of DMD patients. The tools such as Vignos scale and Brooke scale reflect the function of lower extremities and upper extremities separately and therefore they are not enough to check the change in comprehensive functions of DMD patients. The MDFRS has weakness that it requires lots of time and effort to apply it patients. Functional evaluation tools that can reflect the condition of DMD patients comprehensively and objectively and are easy to use have not been reported yet. This study showed the limit existing functional evaluation tools have and the development of new functional evaluation tools that can check clinical effect of medical treatment at this stage new remedies make appearances is needed.
The abilities of the Vignos scale for reflecting functional deterioration and the Brooke scale for decreasing ankle PROM as age increases in DMD patients were confirmed in this study. Also the results of exon deletion and duplication in 69 (58.0%) and 11 (9.2%) patients, respectively, resemble that of previous reports on DMD patients. Additionally, despite the statistical significance on the Brooke scale in those with single and 4 and more exons deletion at the 'hot spot' between exon 44 and 55, supporting evidence for its clinical significance is still lacking. Differences in the Vignos scale and the PROM of bilateral ankle dorsi-flexion were not statistically significant.
The study confirmed the limitation of currently used functional evaluation tools in reflecting functional deterioration according to age and only evaluating upper or lower extremity function in DMD patients. A recent increase in life expectancy of DMD patients and large clinical trials on new treatment methods require new functional evaluation tools for DMD patients that can reflect the clinical characteristics and quantitatively judge the treatment effect, as well as accurately represent the functional deterioration as the disease progresses and integrated overall function of the DMD patients, to be developed.
References
1. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010; 9:77–93. PMID: 19945913.

2. Elbrink J, Malhotra SK. The pathogenesis of Duchenne muscular dystrophy: significance of experimental observations. Med Hypotheses. 1985; 17:375–385. PMID: 4046906.

3. Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatr Neurol. 2007; 36:1–7. PMID: 17162189.

4. Hoogerwaard EM, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, Leschot NJ, Van Essen AJ, Brunner HG, van der Wouw PA, Wilde AA, et al. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in The Netherlands: a cohort study. Lancet. 1999; 353:2116–2119. PMID: 10382696.

5. Moon JH, Park YG, Park JS, Na YM, Kim YJ, Kang SW. Clinical profile of Duchenne muscular dystrophy. J Korean Acad Rehabil Med. 2001; 25:241–248.
6. Madorsky JG, Radford LM, Neumann EM. Psychosocial aspects of death and dying in Duchenne muscular dystrophy. Arch Phys Med Rehabil. 1984; 65:79–82. PMID: 6696607.
7. Karol LA. Scoliosis in patients with Duchenne muscular dystrophy. J Bone Joint Surg Am. 2007; 89(Suppl 1):155–162. PMID: 17272432.

8. Sasaki K, Sakata K, Kachi E, Hirata S, Ishihara T, Ishikawa K. Sequential changes in cardiac structure and function in patients with Duchenne type muscular dystrophy: a two-dimensional echocardiographic study. Am Heart J. 1998; 135:937–944. PMID: 9630096.

9. Romfh A, McNally EM. Cardiac assessment in Duchenne and Becker muscular dystrophies. Curr Heart Fail Rep. 2010; 7:212–218. PMID: 20857240.

10. Muntoni F. Cardiomyopathy in muscular dystrophies. Curr Opin Neurol. 2003; 16:577–583. PMID: 14501841.

11. Finsterer J. Cardiopulmonary support in Duchenne muscular dystrophy. Lung. 2006; 184:205–215. PMID: 17006747.

12. Houde S, Filiatrault M, Fournier A, Dube J, D'Arcy S, Berube D, Brousseau Y, Lapierre G, Vanasse M. Deflazacort use in Duchenne muscular dystrophy: an 8-year follow-up. Pediatr Neurol. 2008; 38:200–206. PMID: 18279756.

13. Angelini C, Pegoraro E, Turella E, Intino MT, Pini A, Costa C. Deflazacort in Duchenne dystrophy: study of long-term effect. Muscle Nerve. 1994; 17:386–391. PMID: 8170484.

14. Eagle M. Report on the muscular dystrophy campaign workshop: exercise in neuromuscular diseases Newcastle, January 2002. Neuromuscul Disord. 2002; 12:975–983. PMID: 12467755.

15. Bach JR, Martinez D, Saulat B. Duchenne muscular dystrophy: the effect of glucocorticoids on ventilator use and ambulation. Am J Phys Med Rehabil. 2010; 89:620–624. PMID: 20647779.
16. Guglieri M, Bushby K. Molecular treatments in Duchenne muscular dystrophy. Curr Opin Pharmacol. 2010; 10:331–337. PMID: 20434401.

17. Wang Z, Chamberlain JS, Tapscott SJ, Storb R. Gene therapy in large animal models of muscular dystrophy. ILAR J. 2009; 50:187–198. PMID: 19293461.

18. Nelson SF, Crosbie RH, Miceli MC, Spencer MJ. Emerging genetic therapies to treat Duchenne muscular dystrophy. Curr Opin Neurol. 2009; 22:532–538. PMID: 19745732.

19. Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981; 4:186–197. PMID: 7017401.

20. Lue YJ, Su CY, Yang RC, Su WL, Lu YM, Lin RF, Chen SS. Development and validation of a muscular dystrophy-specific functional rating scale. Clin Rehabil. 2006; 20:804–817. PMID: 17005504.

21. Bakker JP, De Groot IJ, Beelen A, Lankhorst GJ. Predictive factors of cessation of ambulation in patients with Duchenne muscular dystrophy. Am J Phys Med Rehabil. 2002; 81:906–912. PMID: 12447089.

22. Main M, Mercuri E, Haliloglu G, Baker R, Kinali M, Muntoni F. Serial casting of the ankles in Duchenne muscular dystrophy: can it be an alternative to surgery? Neuromuscul Disord. 2007; 17:227–230. PMID: 17303425.

23. Rose KJ, Burns J, Wheeler DM, North KN. Interventions for increasing ankle range of motion in patients with neuromuscular disease. Cochrane Database Syst Rev. 2010; 2:CD006973. PMID: 20166090.

24. Parreira SL, Resende MB, Della Corte Peduto M, Marie SK, Carvalho MS, Reed UC. Quantification of muscle strength and motor ability in patients with Duchenne muscular dystrophy on steroid therapy. Arq Neuropsiquiatr. 2007; 65:245–250. PMID: 17607422.

25. Palmieri B, Sblendorio V, Ferrari A, Pietrobelli A. Duchenne muscle activity evaluation and muscle func tion preservation: is it possible a prophylactic strategy? Obes Rev. 2008; 9:121–139. PMID: 18034791.
26. Heller KD, Wirtz DC, Siebert CH, Forst R. Spinal stabilization in Duchenne muscular dystrophy: principles of treatment and record of 31 operative treated cases. J Pediatr Orthop B. 2001; 10:18–24. PMID: 11269806.

27. Cheuk DK, Wong V, Wraige E, Baxter P, Cole A, N'Diaye T, Mayowe V. Surgery for scoliosis in Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2007; 1:CD005375. PMID: 17253553.

28. Kinali M, Messina S, Mercuri E, Lehovsky J, Edge G, Manzur AY, Muntoni F. Management of scoliosis in Duchenne muscular dystrophy: a large 10-year retrospective study. Dev Med Child Neurol. 2006; 48:513–518. PMID: 16700946.

29. Matecki S, Topin N, Hayot M, Rivier F, Echenne B, Prefaut C, Ramonatxo M. A standardized method for the evaluation of respiratory muscle endurance in patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2001; 11:171–177. PMID: 11257474.

30. Phillips MF, Quinlivan RC, Edwards RH, Calverley PM. Changes in spirometry over time as a prognostic marker in patients with Duchenne muscular dystrophy. Am J Respir Crit Care Med. 2001; 164:2191–2194. PMID: 11751186.

31. Corrado G, Lissoni A, Beretta S, Terenghi L, Tadeo G, Foglia-Manzillo G, Tagliagambe LM, Spata M, Santarone M. Prognostic value of electrocardiograms, ventricular late potentials, ventricular arrhythmias, and left ventricular systolic dysfunction in patients with Duchenne muscular dystrophy. Am J Cardiol. 2002; 89:838–841. PMID: 11909570.

32. Mohyuddin T, Jacobs IB, Bahler RC. B-type natriuretic peptide and cardiac dysfunction in Duchenne muscular dystrophy. Int J Cardiol. 2007; 119:389–391. PMID: 17079036.

33. Beggs AH, Koenig M, Boyce FM, Kunkel LM. Detection of 98% of DMD/BMD gene deletions by polymerase chain reaction. Hum Genet. 1990; 86:45–48. PMID: 2253937.

34. Sellner LN, Taylor GR. MLPA and MAPH: new techniques for detection of gene deletions. Hum Mutat. 2004; 23:413–419. PMID: 15108271.

35. Nicholson LV, Johnson MA, Bushby KM, Gardner-Medwin D, Curtis A, Ginjaar IB, den Dunnen JT, Welch JL, Butler TJ, Bakker E, et al. Integrated study of 100 patients with Xp21 linked muscular dystrophy using clinical, genetic, immunochemical, and histopathological data. Part 2. Correlations within individual patients. J Med Genet. 1993; 30:737–744. PMID: 8411068.

36. Den Dunnen JT, Grootscholten PM, Bakker E, Blonden LA, Ginjaar HB, Wapenaar MC, van Paassen HM, van Broeckhoven C, Pearson PL, van Ommen GJ. Topography of the Duchenne muscular dystrophy (DMD) gene: FIGE and cDNA analysis of 194 cases reveals 115 deletions and 13 duplications. Am J Hum Genet. 1989; 45:835–847. PMID: 2573997.
37. Roberts RG, Bobrow M, Bentley DR. Point mutations in the dystrophin gene. Proc Natl Acad Sci U S A. 1992; 89:2331–2335. PMID: 1549596.

38. Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987; 50:509–517. PMID: 3607877.

39. Forrest SM, Cross GS, Flint T, Speer A, Robson KJ, Davies KE. Further studies of gene deletions that cause Duchenne and Becker muscular dystrophies. Genomics. 1988; 2:109–114. PMID: 3410474.

40. Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988; 2:90–95. PMID: 3384440.

41. Cho H, Hong JM, Lee KA, Choi YC. Clinical usefulness of molecular diagnosis in dystrophin gene mutations using the multiplex ligation-dependent probe amplification (MLPA) method. J Korean Neurol Assoc. 2010; 28:22–26.
Fig. 1
The Brooke scale (A), and Vignos scale (B) of Duchenne muscular dystrophy patients with age. A score of 10 on the Vignos scale means dead (*p<0.001).
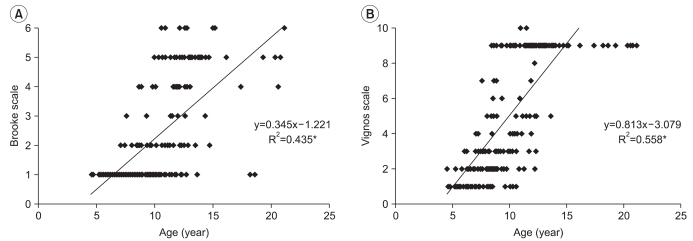
Fig. 2
Correlation between age and right ankle dorsi-flexion PROM (A), and left ankle dorsi-flexion PROM (B), PROM: Passive range of motion (*p<0.001).

Fig. 3
Bilateral muscle power (N) with age, right (A) and left (B) shoulder abductor, right (C) and left (D) knee extensor.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download