Abstract
Objective
To assess the efficacy of human placental extract (HPE) in an animal model of rheumatoid arthritis (RA).
Method
We used (i) KRN C57BL/6 TCR transgenic x NOD mice (KBx/N) serum transfer arthritis and (ii) collagen-induced arthritis (CIA) mice to evaluate the effi cacy of HPE (1 ul or 100 ul, intra-peritoneal, three times per week) on RA. Incidence, severity of arthritis, and hind-paw thickness were quantifi ed. Joint destruction was analyzed using modifi ed mammographic imaging. Histopathological analysis for inflammation, cartilage, and osteoclasts was performed using Hematoxylin-eosin (H-E), safranin-O, and tartrate-resistant acidic phosphatase (TRAP). ELISAs were used for detection of various cytokines in serum and joint tissue.
Results
There were no significant differences in incidence of arthritis, clinical scores of arthritis, and hind-paw thickness between HPE-treated and vehicle-treated groups for up to 2 weeks in the KBx/N serum transfer arthritis model. Histopathological analysis also showed no differences 2 weeks after treatment. Levels of TNF-α, IL-1β, IL-6, IL-10, and RANKL in serum and joint tissues were similar in all groups. Furthermore, there were no differences in clinical, radiological, and histological parameters between HPE-treated and vehicle-treated group for 3 weeks in the CIA model.
Recently, injections using human placental extract (HPE) have been used extensively.1 HPE has various effects including regulation of the autonomic nervous system and the endocrine system, enhancement of immune functions, anti-inflammation, elimination of free radicals, facilitation of wound recovery, liver detoxication, fatigue recovery, and increase in appetite.2 However, animal experiments and clinical studies on HPE are insufficient to scientifically demonstrate a working mechanism, an effect on RA, and side effects. In practice, the use of HPE has been approved by the Korean Food and Drugs Administration only for 'improvement of menopausal disorders' and 'liver function improvement and fatigue recovery.' However, cases where HPE has been applied in Korea and Japan show that it is considered a panacea, and has been applied to improve liver function, and to treat of menopausal disorders, menstrual irregularity, menstrual pain, infertility, vaginitis, genital insufficiency, asthenia, dementia, headache, low back pain, osteoarthritis, frozen shoulder, atopic dermatitis, to improve sexual function and as therapy for alopecia.2,3
Rheumatoid arthritis (RA) is a systemic chronic inflammatory disease as well as a representative autoimmune disorder.4 There are 2 possibilities regarding the effect of HPE on RA; it may either improve or exacerbate rheumatoid arthritis. First, HPE may improve rheumatoid arthritis since it has an anti-inflammatory effect as well as the ability to eliminate free radicals and to recover from and reconstruct wounds. Some studies actually showed that HPE increased growth of chondrocytes and repressed damage to cartilage.5,6 Experiments performed using an inflammatory arthritis animal model showed that local injection of HPE reduced the symptoms of arthritis and the expression of inflammatory cytokines.7 In prior studies that were conducted, some patients with rheumatoid arthritis verified that placenta-eluted gamma globulins have a therapeutic effect.8,9 On the other hand, it is known that the placenta contains various growth factors such as hepatocyte growth factor (HGF), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), placenta growth factor (PlGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), insulin like growth factor (IGF) and transforming growth factor (TGF).2 Among these, VEGF and PIGF are particularly well known as representative substances that exacerbate rheumatoid arthritis,10-13 supporting the possibility that HPE may exacerbate rheumatoid arthritis. However, no study has been conducted on the systemic injection of HPE on rheumatoid arthritis.
Therefore, we investigated the effect of systemic HPE injection on rheumatoid arthritis using two representative animal models for rheumatoid arthritis: (i) a KBx/N with serum transfer arthritis and (ii) a collagen-induced arthritis (CIA) model.
In this study, we used HPE provided by Melsmon (Melsmon, Tokyo, Japan). HPE is a liquid agent prepared by freezing the villous tissue part of fresh placenta and then extracting and purifying it by means of hydrochloric acid hydrolysis. The extract contains 100 mg of water-soluble substances in 2 ml of normal saline solution. The identified components are nucleic acid related substances such as uracil, adenine, guanine and thymine, amino acids such as lysine, alanine, asparagic acid, leucine, glutamic acid, glycine, valine, serine, tyrosine, phenylalanine, threonine, arginine, proline, cystine, methionine, histamine, and minerals such as sodium, potassium, magnesium, phosphorus, iron. In this study, a stock solution of the HPE agent was diluted with normal saline and injected intraperitoneally.
For the preparation of the KBx/N serum transfer arthritis model, KRN TCR transgenic mice provided by D. Mathis and C. Benoist (Harvard Medical School, Boston, USA) were first mated with non-obese diabetic (NOD) mice. Arthritis began to occur about four weeks after birth in KBx/N mice. Blood samples were taken in the eighth week when the arthritis was most severe. Serum was separated by centrifugation and stored frozen. BALB/c male mice (Orient Bio Inc., Sungnam, Korea) aged 7-9 weeks were purchased and grown. KBx/N serum (150 ul) was intraperitoneally injected into BALB/c male mice two times on Day 0 and Day 2 to induce arthritis. Experimental animals were divided into three groups: Group 1 (n=10) was the control group, to which vehicle was administered; Group 2 (n=10) received 1 ul of HPE; Group 3 (n=10) received 100 ul of HPE. Administration of 1 ul HPE was comparable to a dose of 2 ml, which is the recommended therapeutic dose for humans. HPE 100 ul was the maximum dose that did not lead to any deaths or fatal toxicity in the mice in a preliminary test, and we gave it as a one-time dose in this experiment. Intraperitoneal injection of 1 ul or 100 ul HPE and vehicle was given two times per week to all mice, from Day 0 to day 14.
For the preparation of the CIA model, DBA/1 male mice (Orient Bio Inc., Sungnam, Korea) aged 7-9 weeks were purchased. Bovine-type type II collagen (Chondrex inc., Redmond, USA) (150 µg) and the same quantity of complete Freund's adjuvant (CFA) (Chondrex inc., Redmond, USA) were injected intradermically through the tail. Twenty one days later, bovine-type type II collagen (150 µg) and the same quantity of incomplete Freund's adjuvant (IFA, Chondrex) were injected through the tail to induce arthritis. The experimental animals were divided into two groups. Group 1 (n=8), which was the control group, was administered vehicle; Group 2 (n=8) received 1 ul of HPE. Intraperitoneal injection of 1 ul HPE and vehicle was given two times per week to all mice from Day 21 to the end of the experiment.
The incidence and severity of arthritis were assessed macroscopically by observing mice two or three times per week. The severity of arthritis was coded as Grades 0 to 3 for each leg: Grade 0, without any edema or swelling; Grade 1, mild edema and flare localized to the toes or ankle joints; Grade 2, moderate edema and flare over the ankle joints and toes; and Grade 3, severe edema and flare over the region between the ankle joints and the entire toes or accompanied by joint stiffness. The assessment was performed with reference to the sum of the severity measured from the four legs, and thus the maximum possible arthritis score was 12 points. The presence of arthritis was defined as definite arthritis accompanying flare and edema together. The percentage (%) of the legs where arthritis occurred among all the legs of the subject mouse was recorded as the incidence of arthritis. The thickness of the two rear feet was measured at the thickest part of the ankles for all mice using electronic calipers (Orient Bio Inc., Sungnam, Korea). To minimize rater error, arthritis assessment was done by two raters who were not aware which were the experimental and which the control groups.
A mixture of Zoletil (Virbac, Magny-en-Vexin, France) and Rumpun (Bayer, Seoul, Korea) was diluted ten times with phosphate buffered saline (PBS) and 200 ul of the solution was intraperitoneally injected into the mice to anesthetize them. Blood samples were then taken by cardiocentesis. Serum was separated by centrifugation of the blood samples and stored at -70℃ before the test. Ankle joint tissues were taken after removing the skin, rapidly cooling them with liquid nitrogen, and then storing them frozen at -70℃ until use. Ankle tissues were ground in a mortar using liquid nitrogen and proteins were extracted with a lysis buffer (Mammalian Protein Extraction Reagent) (Pierce Biotechnology Inc., Rockford, USA) containing proteinase inhibitor cocktail (Calbiochem, San Diego, USA). In the serum and joint tissue extractions, the cytokine concentrations of TNF-α, IL-1β, IL-6, soluble receptor activator of NF-κB ligand (sRANKL) and IL-10 were measured using ELISA kits (PeproTech inc., Rocky Hill, USA). The capture antibody for the cytokine to be measured was put into a 96-well plate (Nunc, Rochester, USA) for sandwich ELISAs and reacted overnight at 4℃. Then, PBS containing the blocking solution (1% bovine serum albumin (BSA)/0.05% Tween-20) was added and reacted for two hours at room temperature. The standard samples and the samples to be measured were reacted for two hours at room temperature. After washing them with water, the detection antibody was added and reacted for two hours at room temperature. Then, Avidin-peroxidase (50 µl, 1 : 2,000) and ABTS substrate solution were added to each of the wells one by one and allowed to react. Following the reaction, light absorbance was measured at 450 nm with an ELISA reader. a standard curve was acquired using the concentrations and absorbances of standard samples. Then the absorbance of the tested samples converted to concentrations using the standard curve. The measurement was repeated for all the tested samples according to the directions of the manufacturer, and the mean values were used for the analysis.
We performed radiologic assessment to investigate the effect of HPE on the degree of bone destruction. Radiological assessments were performed only with the CIA model because the KBx/N model, which is a two-week model, did not show a clear change that could be found by general radiological tests since destruction of bone was relatively weak.
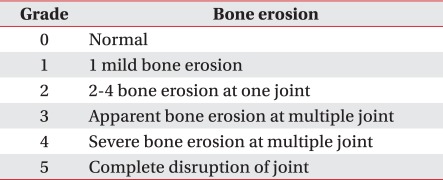
The radiography was performed at the knee and ankle joints of mice 42 days after the induction of arthritis, using a digital mammograph (Mammomat NovationDR) (Siemens, Erlangen, Germany). A full field flat panel digital detector that could create a 24×29 cm image (maximum matrix size, 3,328×4,096; pixel size, 70 µm) was used, and all the images were taken under exposure conditions of 30 kVp, 90 mAs and 1.5 magnification. The destruction of the joints was assessed with respect to the region between the patella and the femur and the region between the femur and the tibia in case of knee joints; and with respect to the three regions of the ankle, the intertarsal part and the metatarsophalangeal joint in the case of rear foot joints. Joints received scores of Grade 0 (normal) to grade 5 (complete disruption of the joint) (Table 1). Mean radiological scores were determined as the mean of the measurements at the respective regions.
Histopathologic assessment was performed 14 days after the induction of arthritis in the KBx/N model and 42 days after induction in the CIA model.
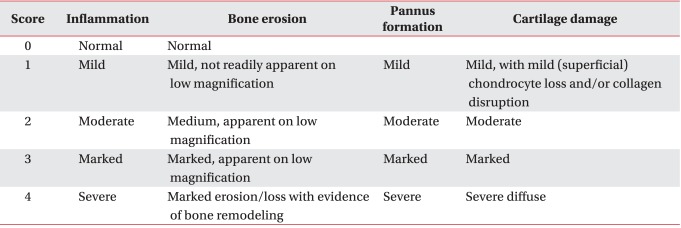
For the histopathologic assessment, we took knee and ankle joints and prepared paraffin blocks through a fixation procedure with formalin and a decalcification procedure with Calci-Clear Rapid (Diagnostics, Atlanta, USA). Blocks were sectioned into slices of 4 µm thickness and attached to slides. After deparaffinizing with xylene three times, for five minutes each time, we washed the slices with distilled water and performed hematoxylin and eosin (H&E), Safranine-O and tartate-resistant acid phosphatase (TRAP) staining. The progress of arthritis was assessed quantitatively with respect to bone erosion, pannus formation and cartilage damage. Scores included in five grades between 0 (normal) and 4 (severe) (Table 2).
In 10 randomly selected zones at 200 X magnification, the number of giant cells containing at least three TRAP-positive nuclei was counted, summed and marked as the total number of TRAP-positive cells.
For comparison of mean values among the groups, a Kruskal-Wallis test was performed in the KBx/N model and a Mann-Whitney U-test in the CIA model. All the results were expressed as 'mean±standard deviation' Statistical analysis of the data was done using SPSS Version 11.0 (SPSS, Chicago, USA). Differences where p was less than 0.05 were considered significant.
There was no significant difference in the incidence of arthritis among the three treatment groups (vehicle, HPE 1 ul, HPE 100 ul). The respective arthritis scores were 9.11±1.96, 10.22±1.71, and 10.22±1.48 for the first week and 5.77±1.30, 6.89±1.90, and 5.89±0.78 in the second week (Fig. 1-A, B). There was also no between group differences in arthritis severity. The thickness of the two ankles measured with the electronic calipers in each of the mice was 2.61 mm, 2.61 mm, and 2.60 mm in the initial stage, 3.59 mm, 3.58 mm, and 3.65 mm in the first week, and 3.06 mm, 3.17 mm, and 3.19 mm in the second week in the respective groups (Fig. 1-C). No significant difference was found in the improvement or exacerbation of arthritis due to HPE. Neither decreased weight nor death was found in the 100 ul HPE group (Fig. 1-D).
For histological assessment, we performed H&E and Safranine-O staining, and quantitatively assessed disease progress in terms of synovial inflammation, cartilage damage, and bone erosion. In the vehicle, HPE 1 ul, and HPE 100 ul groups, the infiltration of synovial inflammation cells was 2.83±0.24, 2.36±0.29, and 2.73±0.41, the cartilage damage was 2.07±0.53, 1.86±0.36, and 1.97±0.29, and the bone erosion was 2.33±0.68, 1.83±0.38, and 2.05±0.45, respectively, indicating that the histological findings were similar to each other (Fig. 2-A, B). TRAP staining was done to assess osteoclasts, playing a key role in bone erosion in rheumatoid arthritis. The results showed no difference in differentiation and total number of osteoclasts (Fig. 2-A, C). Similar to the macroscopic progressive pattern of the arthritis, the histological findings did not show improvement or exacerbation of the arthritis due to HPE in terms of synovial inflammation, cartilage damage and bone erosion.
At the end of the experiment we measured ,in the serum of mice, the concentrations of IL-1β, TNF-α, and IL-6, which are important inflammatory cytokines involved in the occurrence of rheumatoid arthritis, IL-10, which represses chronic inflammation in rheumatoid arthritis, and sRANKL, which is fundamental to the differentiation and activation of osteoclasts. IL-1β and TNF-α were also measured at the ankle joints. Serum concentrations of IL-1β in the respective groups were 235.38±15.11 pg/ml, 221.35±19.35 pg/ml, and 224.03±18.22 pg/ml. IL-10 concentrations were 204.86±36.20 pg/ml, 186.51±22.36 pg/ml, and 212.83±55.06 pg/ml. TNF-α concentrations were 659.60±249.53 pg/ml, 559.30±151.46 pg/ml, and 678.45±278.31 pg/ml. IL-6 levels were 566.90±127.04 pg/ml, 568.80±52.98 pg/ml, and 568.98±144.31 pg/ml, while sRANKL concentrations were was 1,472.62±174.03 pg/ml, 1,323.32±161.28 pg/ml, and 1,410.87±367.16 pg/ml. This indicated that there were no decreases in IL-1β, TNF-α, IL-6, and sRANKL levels and no increases in IL-10 level in groups exposed to HPE. Exacerbation of inflammatory cytokines was also not found (Fig. 3).
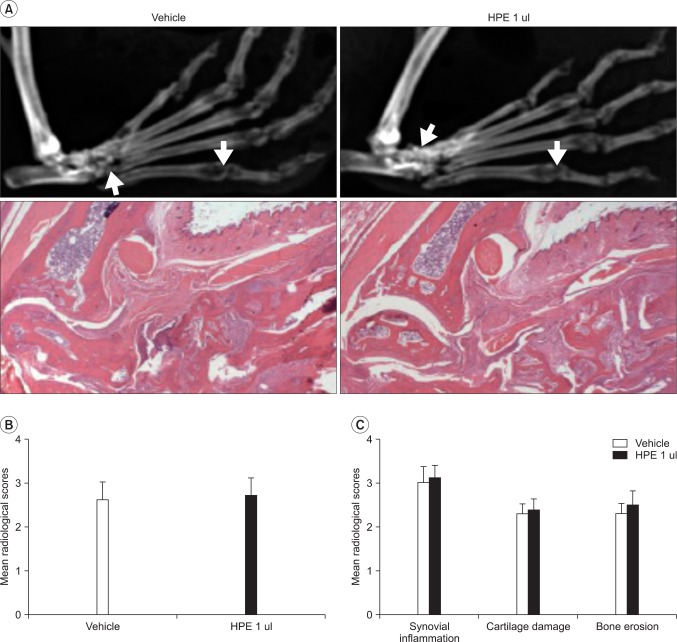
The severity of arthritis was assessed in the CIA model where autoimmune arthritis was induced. The arthritis scores in the vehicle and HPE administration groups were 3.75±2.22 and 4.00±1.83 on the 10th day and 6.04±1.91 and 5.75±1.25 at the end of the experiment, respectively. Similar arthritis severity findings were found for the two groups (Fig. 4-A). The ankle thickness in the respective groups was 2.94±0.18 mm and 2.91±0.11 mm at the beginning of the experiment, 3.31±0.34 mm and 3.58±0.55 mm on the 10th day, and 4.06±0.53 mm and 4.02±0.42 mm at the end of the experiment (Fig. 4-B). No significant difference was found in improvement or exacerbation by HPE between the two groups. Radiography using digital mammography and histopathologic assessment based on HE staining, which were done to assess the effect of HPE on destruction of bones (which is an important treatment goal in rheumatoid arthritis), clearly showed destruction of bone in both control and experimental groups (Fig. 5-A). The mean radiological score of the two groups was 2.67±0.34 and 2.82±0.25, respectively, indicating that bone destruction was mild in both groups (Fig. 5-B). The histological analysis scores in the respective groups were 3.06±0.20 and 3.22±0.36 for synovial inflammation, 2.27±0.33 and 2.32±0.41 for cartilage damage, and 2.29±0.28 and 2.48±0.31 for bone erosion, showing no improvement or exacerbation by HPE (Fig. 5-A, C).
As placental agents containing various constituents are known to have various effects on our bodies, they have been used extensively for treatment of various diseases for a lengthy period of time.2,3
According to the extraction method, HPE may be classified into two types: extraction from villous tissue and hydrolysates derived from the extraction of the whole placenta.14 At present, only extraction from villous tissue is permitted for the improvement of menopausal disorders, and only whole placenta for the improvement of liver functions.15 There were two clinical studies in Korea conducted on the effect of HPE on menopausal disorders. Kong et al.14 reported that HPE improved menopausal symptoms and fatigue in middle aged women when compared with the placebo group. Park et al.15 reported a double blind placebo controlled study in which HPE improved some menopausal symptoms but did not show a significant change in female hormones. It is generally accepted that the mechanism of the improvement of menopausal disorder symptoms by HPE is through the anti-inflammatory effect of HPE.16-18 The anti-inflammatory effect of HPE and the improvement in menopausal disorders suggests the possibility that HPE has a positive effect on rheumatoid arthritis, which is a representative chronic inflammatory disease whose incidence is high in menopausal women.
Rheumatoid arthritis is a systemic inflammatory disease and a representative autoimmune disease characterized by chronic synovial inflammation, local joint destruction and general osteopenia.4 Some studies have investigated changes in the growth of chondrocytes, repression of cartilage damage, reduced arthritis symptoms and inflammatory cytokine expression due to local HPE injection, and the therapeutic effects of placenta-eluted gammaglobulins.5-9 Since rheumatoid arthritis is a disease that is invasive to the general joints, it is important to investigate the effect of general HPE administration. We investigated the effect of general HPE administration using two representative rheumatoid arthritis animal models. In both of the models, HPE did not induce clinical improvement, reduce inflammation, or reverse destruction of cartilage and bone.
Since a placenta includes VEGF, PIGF and TNF-α,10-13 general administration of HPE may exacerbate rheumatoid arthritis. However, the result of our experiment did not show any exacerbation with regard to clinical progress, inflammation, and destruction of cartilage and bone. The result of our study showed that the general administration of HPE did not have any improving or exacerbating effects in rheumatoid arthritis animal models. In other words, it is highly probable that corticotropin-releasing factors or immunoglobulins, which may have a direct inflammatory effect on rheumatoid arthritis or VEGF, PIGF, and TNF-α, which may exacerbate rheumatoid arthritis, might have been increased or decreased in the preparation of the HPE agents. Further experiments may need to be conducted to identify the above-mentioned substances contained in the HPE agents or to investigate the direct effect of the substances contained in the HPE on rheumatoid arthritis.
The recent study entitled, "Analysis about Consumers Use of Human Placental Injection and Development of Public Relations Technique," published by the Korea Food and Drug Administration, showed that 80% of HPE injections involved misuse or abuse of HPE. The purpose of trying HPE injections was recovery from fatigue and improvement of female menopausal symptoms. But this occurred in only 23% and 13% of our subjects, respectively. On the other hand, 41% of instances of HPE use were for skin beauty or whitening; 4% were for pain in the joints.1 One of the reasons why HPE is used indiscreetly is insufficient experimental data and clinical studies on the effect of HPE injections. In practice, the only research data about HPE injections that are available in Korea are related to menopausal disorder symptoms. Some studies dealt with experiments concerning skin whitening. Our study is the only report about the effects of systemic HPE administration on rheumatoid arthritis. Therefore, further systematic and various other studies need to be conducted to investigate the effects of HPE on various diseases.
We assessed the effect of HPE general administration on the incidence rate and severity of rheumatoid arthritis, radio logical bone destruction, cytokine expression in blood and joint tissues, microscopic inflammation findings, cartilage damage, and bone erosions in the KBx/N serum transfer model and the CIA model which are the two representative animal models for rheumatoid arthritis. Systemic HPE administration did not show any effect on the treatment of rheumatoid arthritis in rheumatoid arthritis models involving experimental animals. Therefore, the indiscreet use of HPE for RA should be forbidden.
References
1. Lee ES. Analysis about consumers use of human placental injection and development of public relations technique. 2010. Korea food and drug administration.
2. Sim CU. The placenta therapy. 2005. 1st ed. Seoul: MD World;p. 31.
3. Park NJ. Safety, efficacy and limitations of medical use of placental extract. J Korean Med Assoc. 2005; 48:1013–1021.

4. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003; 423:356–361. PMID: 12748655.

5. Huh J, Suh MS, Park SJ, Lim YK, Shin JH, Chung HY, Cho BC, Park JW. The effect of placenta extract on proliferation and differentiation of human chondrocytes. J Korean Soc Plast Reconstr Surg. 2006; 33:616–620.
6. Kim JK, Kim TH, Park SW, Kim HY, Kim S, Lee S, Lee SM. Protective effects of human placenta extract on cartilage degradation in experimental osteoarthritis. Biol Pharm Bull. 2010; 33:1004–1010. PMID: 20522967.

7. Yeom MJ, Lee HC, Kim GH, Shim I, Lee HJ, Hahm DH. Therapeutic effects of Hominis placenta injection into an acupuncture point on the inflammatory responses in subchondral bone region of adjuvant-induced polyarthritic rat. Biol Pharm Bull. 2003; 26:1472–1477. PMID: 14519957.
8. Combe B, Cosso B, Clot J, Bonneau M, Sany J. Human placenta-eluted gammaglobulins in immunomodulating treatment of rheumatoid arthritis. Am J Med. 1985; 78:920–928. PMID: 2409794.

9. Sany J, Clot J, Bonneau M, Andary M. Immunomodulating effect of human placenta-eluted gamma globulins in rheumatoid arthritis. Arthritis Rheum. 1982; 25:17–24. PMID: 7066033.

10. Lu J, Kasama T, Kobayashi K, Yoda Y, Shiozawa F, Hanyuda M, Negishi M, Ide H, Adachi M. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol. 2000; 164:5922–5927. PMID: 10820274.

11. Mould AW, Tonks ID, Cahill MM, Pettit AR, Thomas R, Hayward NK, Kay GF. Vegfb gene knockout mice display reduced pathology and synovial angiogenesis in both antigen-induced and collagen-induced models of arthritis. Arthritis Rheum. 2003; 48:2660–2669. PMID: 13130487.

12. Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy A, Hooper A, Priller J, Klerck B, et al. Revascularization of ischemic tissues by PIGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002; 8:831–840. PMID: 12091877.
13. Yoo SA, Yoon HJ, Kim HS, Chae CB, De Falco S, Cho CS, Kim WU. Role of placenta growth factor and its receptor flt-1 in rheumatoid inflammation: a link between angiogenesis and inflammation. Arthritis Rheum. 2009; 60:345–354. PMID: 19180491.

14. Kong MH, Lee EJ, Lee SY, Cho SJ, Hong YS, Park SB. Effect of human placental extract on menopausal symptoms, fatigue, and risk factors for cardiovascular disease in middle-aged Korean women. Menopause. 2008; 15:296–303. PMID: 18090035.

15. Park YR, Kim SR, Jeon GH, Kim SH, Chae HD, Kim CH, Suh CS, Lee BS, Choi H, Park HM, et al. Effect and Safety of Human Placental Extract on Menopausal Symptoms. J Korean Soc Menopause. 2009; 15:178–185.
16. Sur TK, Biswas TK, Ali L, Mukherjee B. Anti-inflammatory and anti-platelet aggregation activity of human placental extract. Acta Pharmacol Sin. 2003; 24:187–192. PMID: 12546729.
17. Banerjee KK, Bishayee A, Chatterjee M. Anti-inflammatory effect of human placental extract: a biochemical mechanistic approach. Riv Eur Sci Med Farmacol. 1992; 14:361–366. PMID: 1308603.
18. Shibasaki T, Odagiri E, Shizume K, Ling N. Corticotropin-releasing factor-like activity in human placental extracts. J Clin Endocrinol Metab. 1982; 55:384–386. PMID: 6282924.

Fig. 1
Effects of human placental extract (HPE) on arthritis in the KBx/N serum transfer model. (A) The cumulative incidence of arthritis during the course of the experiment in vehicle (n=10), HPE 1 ul (n=10), and HPE 100 ul (n=10) treatment groups. The severity of arthritis (B), hind-paw thickness measurement (C), and the change of body weight (D) in each group are presented. Values presented are the mean±SEM for each experiment.
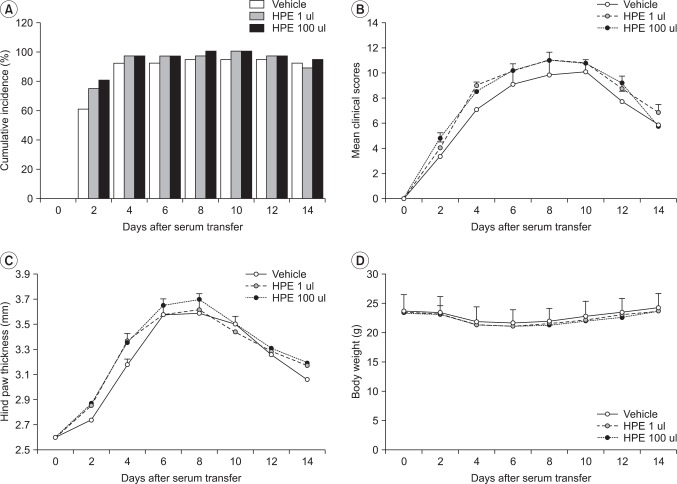
Fig. 2
The histopathological assessment of KBx/N serum transfer arthritic mice treated with human placental extract (HPE). (A) Representative H&E staining (upper row, original magnification ×40), safranin O (middle row, ×40), and TRAP (lower row, ×400) stained joint tissue sections from vehicle (n=10), HPE 1 ul (n=10), and HPE 100 ul (n=10) treatment groups. (B) Histopathological scores of synovial inflammation, cartilage damage, and bone erosion in each group. (C) The total number of TRAP-positive cells in the field was counted in each group. Values presented are the mean±SEM.

Fig. 3
Effects of human placental extract (HPE) on cytokines in sera (A) and joint homogenates (B) of KBx/N serum transfer arthritic mice. Sera and hind paws were collected at the termination of the experiment (day 14) in vehicle (n=10), HPE 1 ul (n=10), and HPE 100 ul (n=10) treatment groups. The concentrations of various cytokines were analyzed by ELISA methods. Values presented are the mean±SEM.
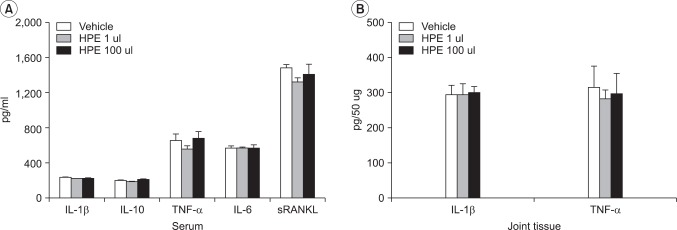
Fig. 4
Effects of human placental extract (HPE) on collagen-induced arthritis (CIA). The severity of arthritis (A) and hind-paw thickness measurement (B) of CIA mice treated with vehicle (n=8) or HPE 1 ul (n=8). Values presented are the mean±SEM for each experiment.
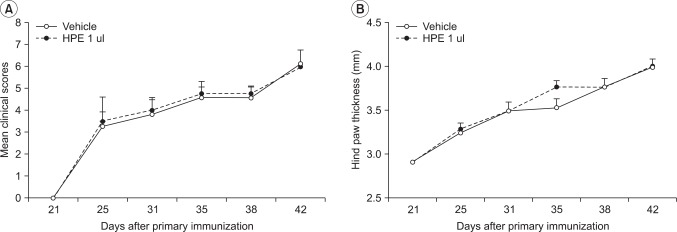
Fig. 5
The radiological and histopathological assessment of CIA mice treated with human placental extract (HPE). (A) Representative radiograph (upper row) and H&E (lower row, original magnification ×40) of vehicle or HPE injected CIA mice. (B) Bone destruction was quantified in knee and ankle joints from CIA mice in each group. (C) Histopathological scores of synovial inflammation, cartilage damage, and bone erosion in each group. Values presented are the mean±SEM.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download