Abstract
Cephalic tetanus is defined as a combination of trismus and paralysis of one or more cranial nerves. Cranial nerves III, IV, VI, VII, and XII may be affected, but the facial nerve is most frequently implicated. A 64-year-old female visited hospital for left ptosis followed by facial palsy after a left forehead abrasion in a car accident. At nine days post injury, left ptosis developed, left facial palsy developed twelve days post injury, and at fifteen days post injury, trismus and dysphagia developed. The following day, there was progression of symptoms to generalized tetanus, such as dyspnea and generalized rigidity. Videofluoroscopic swallow study showed penetration and aspiration. We report a case of cephalic tetanus with ptosis, facial palsy, and dysphagia, which progressed to generalized tetanus.
The incidence and mortality of tetanus have considerably decreased due to an overall improvement in hygiene, vaccination, and administration of antitoxins. Cephalic tetanus accompanied with at least one cranial nerve palsy, in particular, is very rare, accounting for only 0.9-3% of total tetanus cases.1 Thus, not many clinicians are familiar with it. There has been only one case of cephalic tetanus reported in South Korea,2 and no cases have been reported where ptosis and facial palsy accompanied cephalic tetanus before trismus. We experienced a case where cephalic tetanus accompanied with left ptosis and left facial palsy occurred after a left forehead abrasion was diagnosed late, consequently leading to generalized tetanus. Thus, we report the case with a review of the relevant literature to enhance the understanding and diagnosis of the clinical outcome of cephalic tetanus.
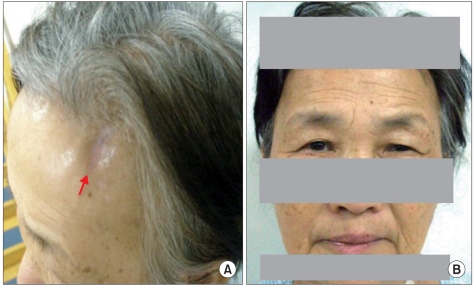
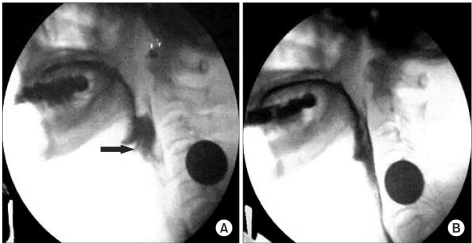
A 64-year-old woman presented with left ptosis and facial palsy that occurred after she sustained a left forehead abrasion (Fig. 1-A) due to a vehicle accident twelve days before her presentation to our hospital. History taking showed that ptosis occurred at nine days after the injury and left facial palsy (Fig. 1-B) at twelve days after the injury without other particular symptoms. Upon her transfer to our hospital from her local hospital, initial assessment showed that her awareness and vital signs were normal. To identify other possible causal diseases or pathological findings, necessary tests such as blood test, urine test, cerebrospinal fluid (CSF) examination, chest radiography, brain computed tomography, and brain magnetic resonance imaging were performed but revealed no abnormal findings. She had no history of tetanus vaccination or tetanus infection. At fifteen days after the injury, trismus (so severe that she could not open her mouth 5 mm or more) and dysphagia occurred, and on the next day, dyspnea and catochus occurred, symptoms aggravated so badly that endotracheal intubation was performed. As cephalic tetanus was suspected, metronidazole for inhibition of active infection and midazolam for control of muscular spasm were administered. However, tetanus immunoglobulin was not administered because it was considered that it was too late to neutralize the toxin. To assess dysphagia at six weeks after the injury, videofluoroscopic swallow study was performed, and penetration and aspiration were detected (Fig. 2-A). After two weeks of rehabilitation for dysphagia, reassessment did not show penetration or aspiration (Fig. 2-B). At eleven weeks after the injury, electrodiagnostic study was performed to assess the left facial palsy. Nerve conduction study of the seventh cranial nerve showed normal compound muscle action potential, and needle electromyography of the craniocervical muscles showed no abnormal spontaneous activity. Blink reflex study, which was recorded on the orbicularis oculi muscle by stimulating the supraorbital nerve, showed no abnormal findings. Since then, symptoms improved and thus the patient was discharged after comprehensive rehabilitation such as electrical stimulation therapy for ptosis and facial palsy and swallowing rehabilitation for dysphasia.
Based on the clinical manifestations of ptosis, facial palsy, and trismus, we considered the possibility of primary cranial nerve palsy and Guillain-Barre syndrome as differential diagnosis in addition to tetanus. At her first presentation, primary cranial nerve palsy was strongly suspected because trismus was not observed. However, as trismus, muscular cramp, and catochus occurred later, the final diagnosis was made as cephalic tetanus that eventually progressed to generalized tetanus. Based on this, we considered that cranial nerve palsy occurred secondary to cephalic tetanus. Guillain-Barre syndrome was also included for differential diagnosis as it may show such symptoms as ptosis, trismus, and dysphagia. However, trismus in Guillain-Barre syndrome is due to weakness of masticatory muscles, thus different from that of tetanus. In this case, the possibility of Guillain-Barre syndrome was excluded because the patient had no weakness of limbs and no particular findings on CSF examination. In addition to Guillain-Barre syndrome, the possibility of other diseases such as Miller-Fisher syndrome, a special subtype of Guillain-Barre syndrome, and myasthenia gravis had to be considered as differential diagnosis.
Tetanospasmin, a toxin produced by Clostridium tetani may cause spastic paralysis of the neuromuscular system. It moves to the neuromuscular junction from the wound, thereafter travels up the axon, finally reaching the central nervous system including the anterior horn cell of the spinal cord.1 It also causes over-activation of the motor system by reducing the secretion of γ-aminobutyric acid and glycine. Besides, it may consequently cause muscle spasms by blocking the excitatory synapses.3 In most cases, tetanus occurs via an external wound. In this case, an elderly patient with low immunity got infected with Clostridium tetani via an abrasion.
Tetanus is classified into generalized, localized, cephalic, and neonatal tetanus.3 In particular, cephalic tetanus may present symptoms such as dysphagia, facial pain, nystagmus, dysarthria, and facial spasm in addition to trismus.1,4 Latent period is about one day to two weeks,5 and characteristically, palsies of cranial nerves III, IV, VI, VII, and XII may be accompanied. Among them, the seventh cranial nerve is most commonly involved, while other cranial nerve palsies are reported to be rare.1,4 In about two-thirds of the patients, tetanus develops into generalized form with mortality of 15-30%.1
In this case, the patient was first diagnosed with palsy of the seventh cranial nerve upon her hospitalization, but was later diagnosed with cephalic tetanus as trismus occurred. In cephalic tetanus, palsy of the seventh cranial nerve is known to occur at the time of or after the onset of trismus.6 However, in this patient's case, diagnosis with tetanus was delayed because facial palsy occurred before the onset of trismus. Regarding this, to the best of our knowledge, there was no case reported in South Korea where facial palsy preceded trismus, but there were five cases where facial palsy occurred prior to trismus in other countries. As such, pathogenesis of cranial nerve palsies and the mechanism of diversity in the sequence of the onset of cranial nerve palsies in tetanus still remains uncertain.7
The reason why the electrodiagnostic study did not show any particular findings was possibly due to the fact that the study was performed when the patient was in recovery phase where the symptoms showed much improvement. However, polyphasic motor unit action potentials that represent the recovery of facial muscles were not clearly observed. Until now, there has been no case where ptosis was accompanied, though such cases were reported in foreign countries. However, according to our investigation on case reports on tetanus that accompanied with ptosis, trismus preceded ptosis in all cases except the case reported by Liu et al.8 In the case report, Liu et al. reported that although the reason why ptosis preceded trismus could not be ascertained, ptosis in tetanus could occur due to palsy of the third cranial nerve or blepharospasm caused by the toxin of Clostridium tetani.8 In tetanus, trismus is the most valuable diagnostic symptom. If ptosis is only observed before trismus occurs, differential diagnosis for other possible diseases is required. In this patient's case, ptosis preceded facial palsy and trismus, and was thus the sole symptom at the early phase of the disease. However, at her presentation to this hospital, physical examination on the function of the extraocular muscle that is innervated by the third cranial nerve was not performed and detailed description of the blepharospasm was not made, thus differentiation of the causes of ptosis was insufficient. The primary principle for the treatment of cephalic tetanus is inhibition of the production of toxins by blocking the spread of infection using antibiotics, and neutralization of toxin using antitoxin. To do this, necrotic tissue of the wound is first removed. Then, antibiotics, tetanus immunoglobulin and an antitoxin are administered to prevent active infection and to neutralize the toxin. At the same time, muscle relaxants or sedatives are administered to control muscle spasm.2 When tetanus is diagnosed, as a rule, tetanus immunoglobulin should be administered as soon as possible regardless of the manifestation of the disease. However, it is known that tetanus immunoglobulin can neutralize only the free circulating toxin that did not reach the nervous system yet, and neurological symptoms progress up to two weeks after the administration of the tetanus immunoglobulin.9 These support the claim that tetanus immunoglobulin cannot neutralize the toxin that already reached nerve cells. In addition, there was one case of cephalic tetanus accompanied with facial palsy prior to trismus reported overseas, where immunoglobulin administered immediately after the progression to generalized tetanus failed to control the symptoms.10
Thus, in this patient's case, we did not administer immunoglobulin considering that it was too late to neutralize the toxin as it had already reached the central nervous system at the time of diagnosis. Even if the immunoglobulin was administered, we believe that the symptoms of generalized tetanus could not have been controlled. Although the immunoglobulin was not administered at an appropriate timing, antibiotics and sedatives were administered appropriately, and rehabilitation program was appropriately performed, thereby the symptoms improved. The patient has been in good condition without complications or relapse till now. Tetanus can be easily diagnosed as it presents trismus first. However, in this patient's case where ptosis and facial palsy occurred without trismus, it was difficult to diagnose cephalic tetanus due to the absence of characteristic symptoms of tetanus such as trismus at the time of the patient's presentation to this hospital, and consequent difficulty in differential diagnosis for other diseases that present cranial nerve palsy. We believe that the administration of immunoglobulin at appropriate timing can prevent the progression to fatal generalized tetanus unless diagnosis is delayed by inclusion of cephalic tetanus in the differential diagnosis for patients with ptosis or facial palsy without trismus. We recognized that it is necessary to consider cephalic tetanus for differential diagnosis when ptosis or facial palsy is the first symptom in patients with head injury. Thus, we report about a case with review to relevant literatures to provide help in the diagnosis of cephalic tetanus that presents symptoms similar to that of this case report.
References
1. Jagoda A, Riggio S, Burguieres T. Cephalic tetanus: a case report and review of the literature. Am J Emerg Med. 1988; 6:128–130. PMID: 3281682.

2. Ryu SH, Seo IY, Park HJ, Oh HK. Cephalic tetanus: a case report. J Korean Assoc Oral Maxillofac Surg. 2004; 30:345–348.
3. Bleck TP. Tetanus: pathophysiology, management, and prophylaxis. Dis Mon. 1991; 37:545–603. PMID: 1874121.

4. Burgess JA, Wambaugh GW, Koczarski MJ. Report of case: reviewing cephalic tetanus. J Am Dent Assoc. 1992; 123:67–70. PMID: 1619169.
5. Park DM. Cranial nerve palsies in tetanus: cephalic tetanus. J Neurol Neurosurg Psychiat. 1970; 33:212–215. PMID: 5443484.

6. Mayo J, Berciano J. Cephalic tetanus presenting with Bell's palsy. J Neurol Neurosurg Psychiatry. 1985; 48:290. PMID: 3981208.

7. Gleeson T, Erienne M. Cranial nerve VII palsy as the first sign of cephalic tetanus after an earthquake. Arch Neurol. 2011; 68:536–537. PMID: 21482936.

8. Liu CY, Liao KK, Fuh JL, Wang PN, Shan DE, Tsai CP. Unilateral blepharospasm as an early sign of cephalic tetanus. Mov Disord. 2009; 24:1094–1095. PMID: 19243061.

9. Smith AT, Drew SJ. Tetanus: A case report and review. J Oral Maxillofac Surg. 1995; 53:77–80. PMID: 7799128.
10. Beecroft CL, Enright SM, O'Beirne HA. Remifentanil in the management of severe tetanus. Br J Anaesth. 2005; 94:46–48. PMID: 15465837.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download