Abstract
Focal myositis is a rare, benign inflammatory pseudotumor of the skeletal muscle of unknown etiology. In Korea, there is no case report of focal myositis, which is not combined with connective tissue disease. We present an unusual case of focal myositis with ankle contracture, involving more than two muscles. A 26-year-old man visited our clinic complaining of right ankle contracture and leg muscle pain. Physical examination revealed no muscle weakness or any other neurological abnormality. T2-weighted magnetic resonance imaging of the right leg demonstrated diffuse high signal intensity of the right gastrocnemius, flexor digitorum longus, and tibialis anterior muscles. Needle electromyography showed profuse denervation potentials with motor unit action potentials of short duration and small amplitude from the involved muscles. All these findings suggested a diagnosis of focal inflammatory myositis and the patient was put under oral prednisolone and physical therapy.
Focal myositis is a rare disease associated with inflammatory changes invading the skeletal muscles. It can be readily confused with tumors or other inflammatory diseases. The etiology of focal myositis has not been elucidated. Nonetheless, the etiology has been speculated to be pertinent to viral infection or the denervation of muscles.1 Special findings are not usually detected in blood tests, and histological tests aid diagnosis. Generally, focal myositis improves spontaneously. But, during the acute phase, pain and complications can be prevented by conservative treatments such as medications and physical therapies.
Recently, the non-invasive technique of magnetic resonance imaging (MRI) has been used as a major evaluation tool.2 The present case reports on a patient who developed ankle contracture without the history of trauma. Focal myositis in the calf that is not associated with rheumatoid arthritis and other connective tissue diseases has not hitherto been reported in Korea. Additionally, the case presented with the very rare condition of the simultaneous invasion of several adjacent muscles.
A 26-year-old man visited our clinic complaining of right ankle contracture, leg muscle pain and gait disturbance that had begun 6 weeks previously. Initial symptom was myalgia in the right calf area, which was worsened by acupuncture in the right calf area 4 weeks prior to the present visit. No past history of trauma, special disease history, or family history was evident.
Upon physical examination, the range of motion of the right ankle joint was limited to -5 degrees of dorsiflexion. Including this dorsiflexion, muscle weakness or sensory disturbance of both extremities was not observed. The circumference of the calf measured at 7 cm distal to the inferior pole of the patella was 40 cm on both the right and left side. Tumor lesions were not palpated, discoloration of the skin was not observed, and pedal pulses of both sides were identical upon palpation. C-reactive protein (CRP) level (7.05 pg/ml) represented a slight elevation from the normal range of 0.00-3.00 pg/ml. Leukocyte values, erythrocyte sedimentation rate, and other inflammation values were normal. The results of general biochemistry tests including muscle enzymes and viral markers were within normal ranges.
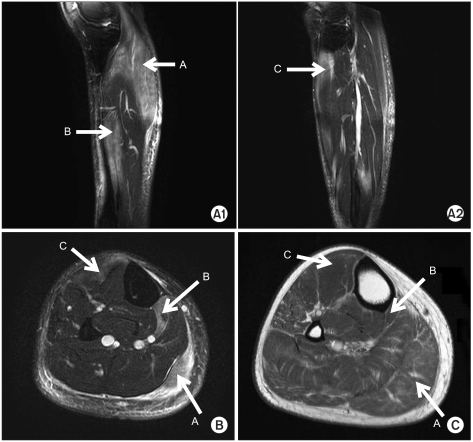
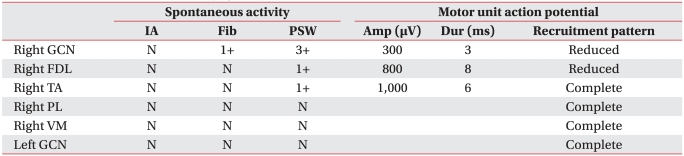
Tibial and fibular plain radiography did not reveal abnormal skeletal findings. A three-phase bone scan revealed increased uptake rate in the right calf area. On T2 weighted MRI, diffuse high signal intensity was detected in the right gastrocnemius muscle, flexor digitorum longus muscle, and anteromedial side of tibialis anterior muscle. T1 weighted images revealed edematous changes in adjacent soft tissues and signs corresponding to ischemic diseases or myositis (Fig. 1). To differentiate vascular diseases, computed tomography angiography (CTA) examination of the lower extremities was performed; no vascular problems in the calf were detected. Electromyography (EMG) was performed, and all nerve conduction results were within normal ranges. Needle EMG in the right gastrocnemius, and the flexor digitorum longus and tibialis anterior muscles revealed abnormal spontaneous activities and small amplitude motor unit action potentials (Table 1).
The collective findings were consistent with a diagnosis of focal myositis. Appropriately, steroid pulse therapy with physical therapy was performed. The oral steroid prednisolone was administered for 14 days by inhalation (60 mg/day, 1 mg/kg, with gradual decrease). A physical examination performed 14 days after initiation of the treatment, revealed improvements in tenderness and pain-related gait disturbance. The previously-limited ankle dorsiflexion was improved from the initial dorsiflexion of -5 degrees to 0 degree.
Focal myositis was described in 1977 for the first time.1 It is classified as an inflammatory myopathy, and it is also referred to as an inflammatory pseudotumor of skeletal muscle or focal nodular myositis. Commonly, the pathogenesis is speculated to be a viral infection or muscle denervation, and the association with the family history or genetic disposition has not been elucidated. Hypotheses concerning the etiology are that it is caused by injury or infection, and that it is a locally developed form of rheumatoid arthritis, sarcoidosis, connective tissue diseases, and other causes.3
Our case developed the symptoms without a past history of injury or infection. In the initial period after onset, the patient was treated with acupuncture, and the symptoms became worse afterward. The location of the lesion as definitely diagnosed by MRI was coincident with the acupuncture site described by the patient. Infection caused by acupuncture was possible but remained unproven based on the sequence of events.
According to previously-reported statistics of 115 focal myositis patients, age ranged from 7-94 years, with no gender difference.3 Focal myositis typically occurs in the skeletal muscles of the lower extremities, but other locations including the head and neck region have been reported in the literature. Nevertheless, among muscles, as shown in our case, the myositis most frequently invades the gastrocnemius, vastus lateralis, and adductor muscles.
Focal myositis can be classified according to the morphologic description of the extent of involvement. Involvement of part of a muscle constitutes type 1, involvement of a whole muscle constitutes type 2, and involvement of two or more muscles in the same compartment or in adjacent compartment is classified as type 3.4 Our case corresponded to type 3, of which the incidence was lower than the other types.
Different from early polymyositis, systemic symptoms, fever, weight loss, and general muscle weakness are rare in focal myositis. Focal myositis may be described as a localized polymyositis. Indeed, focal myositis progresses to dermatomyositis in some cases.5 Nonetheless, they are very rare cases. In addition, different from tumors, focal myositis appears not to invade the fascia, tendons, or adjacent skin.
Generally, blood tests are within normal ranges in most cases, including the present case, and, if muscle enzymes are elevated or abnormal findings of other tests are apparent, consideration of the possibility of polymyositis should prompt testing for other lesions. Similarly, in the present case, systemic syndrome was not observed, and during 6 months follow-up observation, it did not progress to other diseases. Nonetheless, in the future, long-term follow-ups for the development of polymyositis may be required.
Radiographs are normal or reveal only a nonspecific soft tissue mass. On MRI, the enlargement of involving muscles and surrounding edema are observed, although some cases present a normal appearance. Lesions are isointense relative to skeletal muscle on spin echo T1-weighted images and are usually hyperintense on spin echo T2-weighted and short T inversion recovery sequences. Homogeneous enhancement is observed after intravenous administration of gadolinium. In cases with fibrotic change, relatively low signal intensity is shown on all spin-echo pulse sequences.2 In our case, similarly, radiography was normal. Nonetheless, on T2-weighted images, typical findings of the corresponding muscles showing high signal intensity were observed.
In rare cases, relatively low signal intensity on each image that is indicative of the diffuse involvement of the muscles without definite masses needs to be differentiated from collagenous lesions, such as fibromatosis, and from hemosiderin-laden lesions, such as chronic hematoma or giant cell tumors of the tendon sheath.
Sensitivity of MRI, particularly on fat suppression images, is excellent in cases with inflammation and edema. Thus, the technique is useful, not only for the diagnosis of focal myositis, but also in the evaluation of treatment outcomes. In myopathy, MRI shows the change of signal intensity caused by muscle edema or fat deposition, and the change of muscle size or shape from muscle atrophy or pseudohypertrophy. Based on such changes, MRI could be applied to the initial diagnosis, evaluation of number and range of lesions, biopsy site selection in active diseases, and treatment outcome monitoring.6 Focal myositis cases utilizing MRI with a 2-month interval have identified affected muscles and enabled monitoring of treatment.6 Considering the expense of the test, it is questionable whether the cost effect of short-term MRI test is acceptable. Presently, since symptoms were improved after treatments, additional tests were not performed. Nevertheless, if the effectiveness of treatments is not clear even after long-term treatments, for cases requiring differential diagnosis and, thus, biopsy, or for cases requiring surgical treatments because of deformity or contracture, repeated MRI examinations may be of help.
On EMG, the spontaneous activity of the involved muscles display myopathic changes,7 as in the present case. If disease progresses to chronic recurrent myositis, it could show normal or abnormal spontaneous activities related with denervation.
Focal myositis resolves spontaneously within a few months to a few years, but rarely can recur. Steroid or nonsteroidal anti-inflammatory drugs are commonly used for treatment. In progressive diseases, immunosuppressive therapy or radiation therapy may be useful. Nonetheless, the high risk of side effects should be considered.8 Garcia et al.9 reported cases who did not respond to oral steroids but cured by methotrexate. Chiba et al.6 treated by the arterial injection of a low dose of steroid into the proximal artery of the lesion. In our case, symptoms were improved without complications by the non-invasive administration of high dose steroids for a short period.
If the the motion of adjacent joints is limited due to complications of focal myositis, physical therapy and surgical therapy may be required. Maynie et al.10 performed resection of lesions and tenotomy to restore the normal gait of a pediatric patient, who developed equinus deformity from muscle sclerosis due to focal myositis in gastrocnemious. In our case, with medications and physical therapy for 2 weeks, the limitation of dorsiflexion movement was improved from the initial -5 degrees to 0 degree, and the gait pattern normalized.
Focal myositis is often misdiagnosed as tumors resulting in excessive treatments, and may induce joint contracture with progression.10 Thus, appropriate diagnosis and treatments are necessary.
References
1. Manickavasagam J, Majumdar S, Bhattacharyya AK. Focal inflammatory myositis of the paraspinal neck muscles. Ear Nose Throat J. 2008; 87:430–432. PMID: 18712690.

2. Llauger J, Bagué S, Palmer J, Matías-Guiu X, San Román L, Doncel A. Focal myositis of the thigh: unusual MR pattern. Skeletal Radiol. 2002; 31:307–310. PMID: 11981609.

3. Auerbach A, Fanburg-Smith JC, Wang G, Rushing EJ. Focal myositis: a clinicopathologic study of 115 cases of an intramuscular mass-like reactive process. Am J Surg Pathol. 2009; 33:1016–1024. PMID: 19363438.
4. Gaeta M, Mazziotti S, Minutoli F, Genitori A, Toscano A, Rodolico C, Blandino A. MR imaging findings of focal myositis: a pseudotumour that may mimic muscle neoplasm. Skeletal Radiol. 2009; 38:571–578. PMID: 19255757.

5. Cumming WJ, Weiser R, Teoh R, Hudgson P, Walton JN. Localized nodular myositis: a clinical and pathological variant of polymyositis. Q J Med. 1977; 46:531–546. PMID: 594301.
6. Chiba S, Hatanaka Y, Ohkubo Y, Nonaka M, Kashiwagi M, Imai T, Matsumoto H, Satoh M. Focal myositis: magnetic resonance imaging findings and peripheral arterial administration of prednisolone. Clin Rheumatol. 1999; 18:495–498. PMID: 10638778.

7. Smith AG, Urbanits S, Blaivas M, Grisold W, Russell JW. Clinical and pathologic features of focal myositis. Muscle Nerve. 2000; 23:1569–1575. PMID: 11003793.

8. Kisielinski K, Miltner O, Sellhaus B, Krüger S, Goost H, Siebert CH. Recurrent focal myositis of the peroneal muscles. Rheumatology (Oxford). 2002; 41:1318–1322. PMID: 12422007.

9. Garcia-Consuegra J, Morales C, Gonzalez J, Merino R. Relapsing focal myositis: a case report. Clin Exp Rheumatol. 1995; 13:395–397. PMID: 7554571.
10. Maynié M, Robert H, Eloit S, Arnal C, Dolet C. Focal myositis in children. Apropos of a case. Rev Chir Orthop Reparatrice Appar Mot. 1997; 83:382–386. PMID: 9452814.
Fig. 1
Results of right leg MRI. A1 and A2 display Sagittal T2-weighted images (A1 and A2), transverse T2-weighted image (B) and T1-weighted image (C) demonstrates diffuse high signal intensities of the right gastrocnemius (A with arrow), flexor digitorum longus (B with arrow), tibialis anterior (C with arrow) muscles, and edematous changes of the subcutaneous tissue.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download