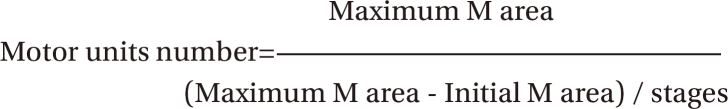1. Di Guglielmo G, Torrieri F, Repaci M, Uncini A. Conduction block and segmental velocities in carpal tunnel syndrome. Electroencephalogr Clin Neurophysiol. 1997; 105:321–327. PMID:
9284240.

2. Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1997; 20:1477–1148. PMID:
9390659.
3. Daube JR. Estimating the number of motor units in a muscle. J Clin Neurophysiol. 1995; 12:585–594. PMID:
8600173.

4. Brown WF. Thenar motor unit estimates in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1973; 36:194–198. PMID:
4708453.
5. McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971; 34:121–131. PMID:
5571599.

6. Koç F, Yerdelen D, Sarica Y, Sertdemir Y. Motor unit number estimation in cases with carpal tunnel syndrome. Int J Neurosci. 2006; 116:1263–1270. PMID:
17000528.
7. Bayrak IK, Bayrak AO, Tilki HE, Nural MS, Sunter T. Ultrasonography in carpal tunnel syndrome: comparison with electrophysiological stage and motor unit number estimate. Muscle Nerve. 2007; 35:344–348. PMID:
17143879.

8. Pino LJ, Stashuk DW, Boe SG, Doherty TJ. Probabilistic muscle characterization using QEMG: application to neuropathic muscle. Muscle Nerve. 2010; 41:18–31. PMID:
19768760.

9. Stålberg E, Nandedkar SD, Sanders DB, Falck B. Quantitative motor unit potential analysis. J Clin Neurophysiol. 1996; 13:401–422. PMID:
8897206.

10. Cuturic M, Palliyath S. Motor unit number estimate (MUNE) testing in male patients with mild to moderate carpal tunnel syndrome. Electromyogr Clin Neurophysiol. 2000; 40:67–72. PMID:
10746180.
11. Werner RA, Albers JW. Relation between needle electromyography and nerve conduction studies in patients with carpal tunnel syndrome. Arch Phys Med Rehabil. 1995; 76:246–249. PMID:
7717817.

12. Dumitru D. Electrodiagnostic medicine. 2002. 2nd ed. Philadelphia: Hanley & Belfus Inc;p. 1058–1070.
13. Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand. 1997; 96:211–217. PMID:
9325471.

14. Stalberg E, Falck B, Sonoo M, Stalberg S, Astrom M. Multi-MUP EMG analysis - a two year experience in daily clinical work. Electroencephalogr Clin Neurophysiol. 1995; 97:145–154. PMID:
7607102.
15. Levine DW, Simmons BP, Koirs MJ, Daltroy LH, Hohl GG, Fossel AH, Katz JN. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993; 75:1585–1592. PMID:
8245050.

16. Bromberg MB. Motor unit estimation: reproducibility of the spike-triggered averaging technique in normal and ALS subjects. Muscle Nerve. 1993; 16:466–471. PMID:
8515754.

17. Doherty TJ, Brown WF. The estimated numbers and relative sizes of thenar motor units as selected by multiple point stimulation in young and older adults. Muscle Nerve. 1993; 16:355–366. PMID:
8455648.

18. Yune YS, Sohn MK, Kim BO. Motor unit number estimation of the thenar muscles. J Korean Acad Rehabil Med. 1997; 21:1184–1193.
19. Boe SG, Stashuk DW, Doherty TJ. Motor unit number estimates and quantitative motor unit analysis in healthy subjects and patients with amyotrophic lateral sclerosis. Muscle nerve. 2007; 36:62–70. PMID:
17455264.

20. Olney RK, Yuen EC, Engstrom JW. Statistical motor unit number estimation: reproducibility and sources of error in patients with amyotrophic lateral sclerosis. Muscle nerve. 2000; 23:193–197. PMID:
10639610.

21. Bayrak AO, Tilki HE, Coşkun M. Sympathetic skin response and axon count in carpal tunnel syndrome. J Clin Neurophysiol. 2007; 24:70–75. PMID:
17277581.

22. Cho SK, Park YK, Lee SC, Moon JH, Min KH, Park YB. Utility of quantitative electromyography in the evaluation of carpal tunnel syndrome. J Korean EMG Electrodiagn Med. 2007; 9:36–42.
23. Miller RG. AAEM minimonograph #28: injury to peripheral motor nerves. Muscle Nerve. 1987; 10:698–710. PMID:
3317034.
24. Sohn MK, Yune YS. Changes of electromyographic signals following peripheral nerve injury. J Korean Acad Rehabil Med. 1997; 21:547–552.
25. Buchberger W. Radiologic imaging of the carpal tunnel. Eur J Radiol. 1997; 25:112–117. PMID:
9283839.

26. Visser LH, Smidt MH, Lee ML. High-resolution sonography versus EMG in the diagnosis of carpal tunnel syndrome. J Neurol Neurochir Psychiatry. 2008; 79:63–67.

27. Kaymak B, Ozçakar L, Cetin A, Candan Cetin M, Akinci A, Hasçelik Z. A comparison of the benefits of sonography and electrophysiologic measurements as predictors of symptom severity and functional status in patients with carpal tunnel syndrome. Arch Phys Med Rehabil. 2008; 89:743–748. PMID:
18374007.

28. Kele H, Verheggen R, Bittermann HJ, Reimers CD. The potential value of ultrasonography in the evaluation of carpal tunnel syndrome. Neurology. 2003; 61:389–391. PMID:
12913205.

29. Park DS, Nam HS, Lee SE, Kim DH, Lee JG, Jeong HO. The usefulness of various electrodiagnostic parameters in mild carpal tunnel syndrome. J Korean Assoc EMG-Electrodiagn Med. 2008; 10:135–143.