INTRODUCTION
MATERIALS AND METHODS
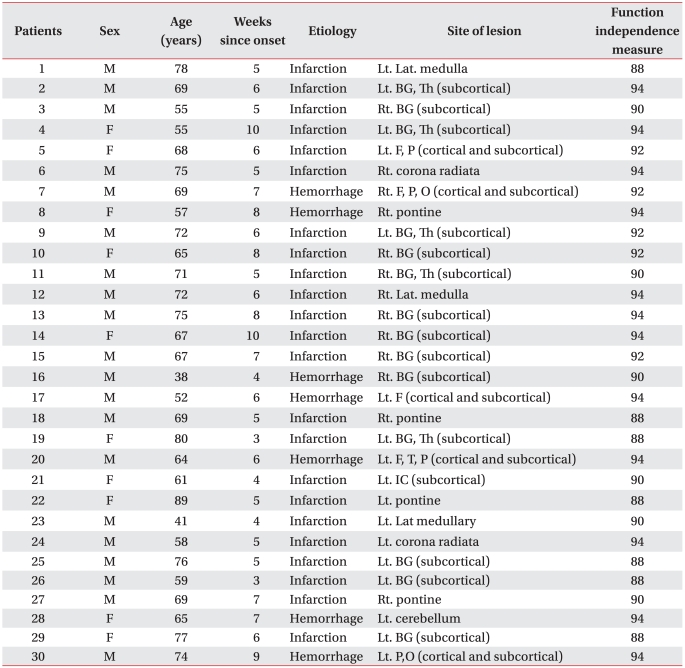
Participants
Experimental design
Experiment I: facilitation of corticospinal excitability during mirror therapy
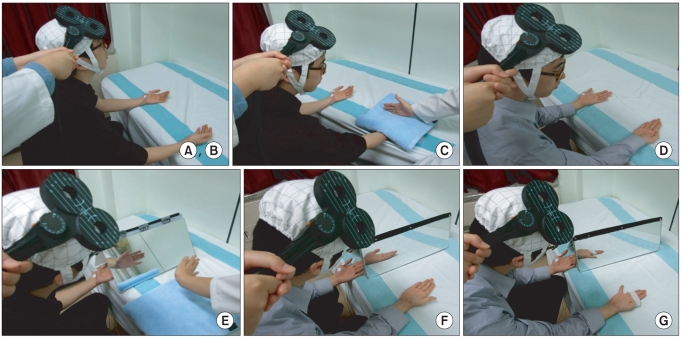 | Fig. 1(A, B) Relaxation and kinesthetic motor imagery state. (C) Observation and imagery of the activity of the hand of another individual without a mirror. (D) Observation and imagery of own hand activity without a mirror. (E) Observation and imagery of the activity of the hand of another individual with a mirror. (F) Observation and imagery of own symmetric hand activity with a mirror. (G) Observation and imagery of own asymmetric hand activity with a mirror. |
For healthy subjects
For stroke patients
Data analysis
RESULTS
Facilitation of corticospinal excitability during motor imagery and mirror therapy in healthy subjects
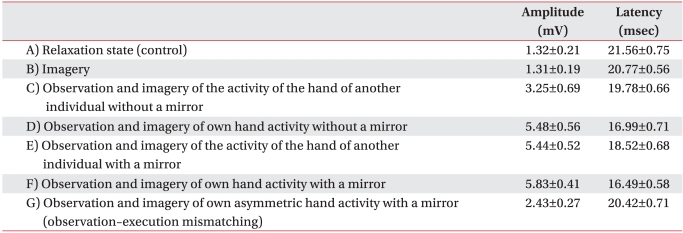
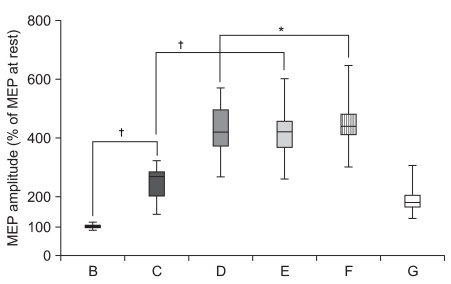 | Fig. 2Increment of percentage MEP values in healthy subjects. Mean (±SEM) values of the MEP amplitude measured during the experiment, expressed as percentage of the MEP at rest in 30 healthy subjects. Based on repeated measures ANOVA, the percentage MEP amplitude of observation and imagery of the activity of the hand of another individual with a mirror (E) was significantly increased compared with observation and imagery of the activity of the hand of another individual without a mirror (C) (†p<0.01). In addition, the percentage MEP amplitude of observation and imagery of self hand activity with a mirror (F) was significantly increased compared with observation and imagery of own hand activity without a mirror (D) (*p<0.05). (A) rest; (B) imagery; (C) observation and imagery of the activity of the hand of another individual without a mirror; (D) observation and imagery of self hand activity without a mirror; (E) observation and imagery of the activity of the hand of another individual with a mirror; (F) observation and imagery of own symmetric hand activity with a mirror; and (G) observation and imagery of own asymmetric hand activity with a mirror. |
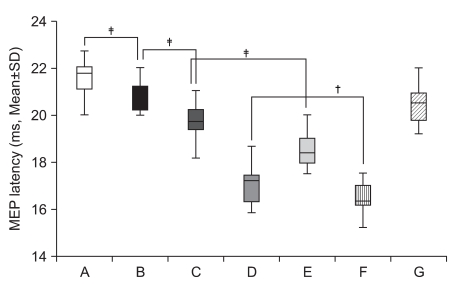 | Fig. 3Decrement of MEP latency values in healthy subjects. Mean (±SEM) values of the MEP latency during the experiment in 30 healthy subjects. Based on repeated measures ANOVA, the MEP latency of observation and imagery of the activity of the hand of another individual with a mirror (E) was significantly decreased compared with observation and imagery of the activity of the hand of another individual without a mirror (C) (‡p<0.001). In addition, the MEP latency of observation and imagery of own hand activity with a mirror (F) was significantly decreased compared with observation and imagery of own hand activity without a mirror (D) (†p<0.01). (A) rest; (B) imagery; (C) observation and imagery of the activity of the hand of another individual without a mirror; (D) observation and imagery of own hand activity without a mirror; (E) observation and imagery of the activity of the hand of another individual with a mirror; (F) observation and imagery of own symmetric hand activity with a mirror; and (G) observation and imagery of own asymmetric hand activity with a mirror. |
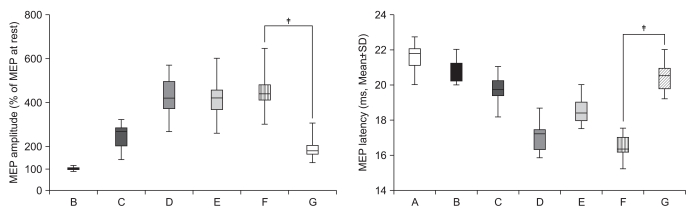 | Fig. 4Observation-execution matching. The percentage MEP amplitude increment and latency decrement of observation and imagery of own symmetric hand activity with a mirror (F) was significantly increased compared with observation and imagery of own asymmetric hand activity with a mirror (G) (‡p<0.001) in 30 healthy subjects (observation-execution matching). |
Facilitation of corticospinal excitability during motor imagery and mirror therapy in stroke patients
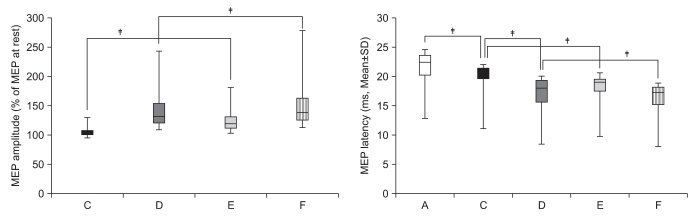 | Fig. 5Increment of percentage MEP amplitude and decrement of MEP latency in stroke patients. The percentage MEP amplitude increment and latency decrement of MEPs were significantly greater in the activity of the hand of another individual with a mirror (E) than in observation and imagery of the activity of the hand of another individual without a mirror (C) (‡p<0.001). In addition, the percentage MEP amplitude increment and latency decrement of MEPs were significantly greater in own hand activity with a mirror (F) than in observation and imagery of own hand activity without a mirror (D) (†p<0.01, ‡p<0.001). (A) rest; (C) observation and imagery of the activity of the hand of another individual without a mirror; (D) observation and imagery of self hand activity without a mirror; (E) observation and imagery of the activity of the hand of another individual with a mirror; (F) observation and imagery of the own hand activity with a mirror. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download