Abstract
Objective
To investigate the injury mechanism in patients who had peroneal neuropathy after a tibio-fibular fracture and the correlation between tibio-fibular fracture location and the severity of the peroneal neuropathy by using electrodiagnosis.
Method
Thirty-four patients with peroneal neuropathy after a tibio-fibular fracture were recruited for this study. Their medical records, radiologic and electrodiagnostic findings were investigated retrospectively. They were divided into 2 groups according to the existence of a fibular head fracture. The group of patients without the fibular head fracture was further classified according to the criteria of Orthopedic Trauma Association (OTA) classification. The differences between the two groups in the severity of the neuropathy and electrodiagnostic findings were evaluated.
Results
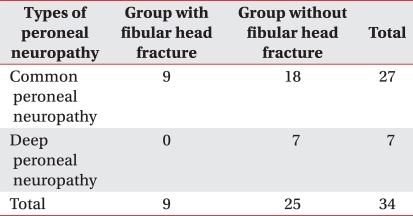
Nine cases (26.5%) had tibio-fibular fractures with a coexisting fibular-head fracture and 25 cases (73.5%) had tibio-fibular fractures without fractures in the fibular-head area. There was no statistical significance in the correlation between the existence of the fibular head fracture and the severity of the electrodiagnostic findings. Neither was there any statistically significant relationship between the site of the tibio-fibular fracture and the severity of the peroneal neuropathy (p>0.05).
Conclusion
This study showed there were numerous cases with common peroneal neuropathy after tibiofibular fracture without a coexisting fibular-head fracture, which shows the importance of indirect nerve injury mechanisms as well as that of direct nerve injury as a cause of peroneal neuropathy. In addition, this study showed that there was no statistically significant correlation between the site of tibio-fibular fracture and the severity of peroneal neuropathy.
It is well known that peroneal neuropathy is the most common mononeuropathy in the lower limb1,2 and it is vulnerable to damage around the fibular head because of the anatomical position between the peroneal nerve and fibula when it winds around the fibular head.3,4 However, the peroneal nerve can be injured anywhere along its course of the leg such as the calf, ankle or foot.5
Clinically, peroneal neuropathy can be suspected when patients complain of foot drop or sensory change of dorsum of their foot, but the differential diagnosis is needed to rule out L4, 5 radiculopathies caused by the herniated nucleus pulposus of the lumbar spine, lumbosacral plexopathy or sciatic neuropathy in which a peroneal division is predominantly damaged.6,7 However, limitations always exists in differential diagnosis by means of only clinical symptoms and physical findings. It is very difficult when a patient complains of atypical symptoms. Therefore, an electrodiagnostic study is used to make the diagnosis.6
These electrodiagnostic findings are closely related with the degree of damage and prognosis, and those resulted by stimulating distal portion of the lesion are different according to the duration from the onset of the nerve damage.8 According to electodiagnostic findings, types of peroneal neuropathy are reported as conduction block, axonal loss or mixed axonal loss/conduction block and the axonal loss type is the most common, reaching 60% of the entire peroneal neuropathies.8
Generally, traumatic tibio-fubular fracture can be suspected as one of the common causes of peroneal neuropathy, but the mechanism of injury is not clearly identified9 and further investigation is required. In this case, there is a strong likelihood that direct laceration and compression were the causes of peroneal neuropathy. However, we consider there can be indirect mechanisms because there have been patients that complained of foot drop after the sole diaphyseal fracture not located closely with the course of the peroneal nerve. If peroneal neuropathy after tibio-fibular fracture can be caused by indirect and direct mechanisms, further analysis will be needed. Therefore, we searched patients diagnosed as peroneal neuropathy by the electrodiagnostic studies and confirmed the existence of tibio-fibular fracture. We investigated fracture site, fracture type and electrodiagnostic findings by their medical records and radiologic findings retrospectively. They were classified into two groups according to the existence of the fracture around the fibular head,10 known as the most vulnerable site of peroneal nerve,9 to find out the injury mechanism of peroneal neuropathy after tibio-fibular fracture. A group without the fibular head fracture was further classified according to the site of the fracture and the degree of severity of the two groups was investigated.
A total of 138 patients that were suspected of peroneal neuropathy for footdrop and diagnosed with common peroneal neuropathy or deep peroneal neuropathy by electrodiagnosis at Kyung-pook National University Hospital rehabilitation center were recruited for this study from January, 2007 to August, 2008. Their medical records, X-ray, CT and MRI findings were investigated retrospectively. A total of 34 patients with traumatic tibio-fibular fractures confirmed by radiologic findings were included. Patients that showed normal findings in electrodiagnosis within 2 weeks from the onset of symptoms were excluded even if they had peripheral nerve injury.11
Medelec Synergy (Oxford Instruments Medical, Surrey, UK) was used for the electrodiagnostic study. Nerve conduction studies and electromyographies were performed.
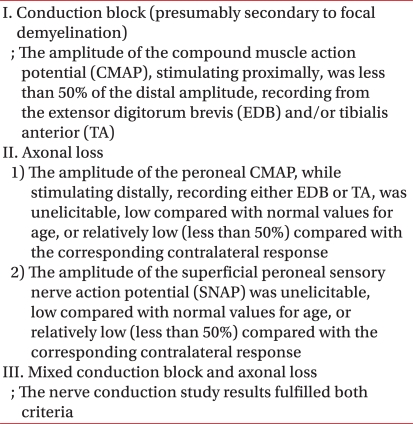
In nerve conduction studies, deep peroneal nerve, tibial nerve, superficial peroneal nerve and sural nerve were performed. In motor nerve conduction studies, active electrodes were placed over the motor point of the muscles and reference electrodes were placed over the tendon or the bony insertion site of the same muscle where active electrodes were placed. In sensory nerve conduction studies, active electrodes were placed over the specific site along the course of a nerve and reference electrodes were placed over a point 4 cm distally apart from the active electrodes. Active electrodes were basically placed at EDB (extensor digitorum brevis) muscle to evaluate deep peroneal nerve. However, in case CMAP (compound muscle action potential) had no response at EDB, we checked it again at the TA (tibialis anterior) muscle. CMAP and SNAP (sensory nerve action potential) were recorded in the nerve conduction studies. In CMAP, baseline to peak amplitude, distal onset latency and nerve conduction velocity were measured. In SNAP, base line to peak amplitude and distal peak latency were measured. According to electodiagnostic findings by Katirji and Willbourn2 (Table 1), types of peroneal neuropathy are reported as conduction block, axonal loss or mixed axonal loss/conduction block. Conduction block was defined as a condition the amplitude of the CMAP, stimulating proximally, was less than 50% of the distal amplitude, recording from EDB and/or TA. Axonal loss was defined as a condition the amplitude of the peroneal CMAP, while stimulating distally, recording either EDB or TA, was unelicitable, low compared with normal values12 for age, or relatively low (less than 50%) compared with the corresponding contralateral response or a condition the amplitude of the superficial peroneal SNAP was unelicitable, low compared with normal values for age, or relatively low (less than 50%) compared with the corresponding contralateral response. Mixed axonal loss/conduction block was defined as a condition that fulfilled both criteria of conduction block and axonal loss. Patients were classified into two groups by severity of peroneal neuropathy: group with complete lesion or incomplete lesion. Complete lesion was defined as a condition which CMAP and SNAP could not be observed in nerve conduction studies or MUAP (motor unit action potential) could not be observed in electromyography, and incomplete lesion was defined as a condition in which MUAP could be observed in peroneal nerve-innervated muscles in electromyography.13 Severity was classified as complete lesion or incomplete lesion because incomplete lesions were expected to have better prognosis than complete lesions because the reinnervation of muscle fibers through axonal sprouting occurred more efficiently in incomplete lesions.14
Needle electromyographies were performed at more than two muscles innervated by deep peroneal nerve such as EDB, EHL (extensor hallucis longus) including TA, peroneus longus innervated by superficial peroneal nerve, biceps femoris short head innervated by common peroneal division of sciatic nerve, tensor fascia lata and gluteus medius innervated by L5 nerve root and gluteus maximus and gastrocnemius innervated by S1 nerve root.
Tibia/fibula fracture classification was carried out by Orthopedic Trauma Association (OTA classification).15 OTA classification classified the tibio-fibular fracture into proximal segment, diaphyseal segment, distal segment or malleolar segment according to their site of fracture (Fig. 1). Proximal and distal segment fractures are further classified into A, B or C types; A type for the group that did not involve the joint, B type for the group that involved the joint partially, and C type for the group that involved the joint entirely. Diaphyseal segment fracture was classified into A, B or C according to fracture patterns: A for simple type, B for wedge type, C for complex type. Malleolar segment fracture was classified into A, B or C according to relative location of fracture based on syndesmosis. A type for fracture below the syndesmosis, B type for fracture at the syndesmosis, C type for fracture above the syndesmosis. Also, all patients were classified into 2 groups according to the existence of fibular head fracture, then severity and electrodiganostic findings of 2 groups were compared to find out the role of the fibular head fracture upon the peroneal nerve injury.
We used the SPSS 12.0 program. Pearson's chi-square test was used to evaluate the relationship between the existence of the fibular head fracture and the electrodiagnostic findings and between the existence of the fibular head fracture and the severity of peroneal neuropathy. Additionally, among the patients without fibular head fracture, the same test was used to evaluate the relationship between the site of tibio-fibular fracture according to the OTA classification and the electrodiagnostic findings and between the site of tibio-fibular fracture and the severity of peroneal neuropathy. All of the statistical significance level was set as p=0.05.
Fifty-eight patients out of a total 138 patients diagnosed as peroneal neuropathy by electrodiagnosis were confirmed to have tibio-fibular fracture with radiologic findings. Seventeen patients with co-existent femur fracture and 7 patients with coexistent pelvic bone fracture were excluded. Therefore, 34 patients without coexistent fracture were included in the study. Subjects included 30 male patients (88.2%) and 4 female patients (11.8%). Mean age was 39.2 (ranging from 12 to 71). Fifth decade were the most common (9 patients, 26.4%) followed by the third and fourth decade (7 patients, 20.6%, respectively). 30 patients (87.9%) were unilateral, 17 patients were left side and 13 patients were right side.
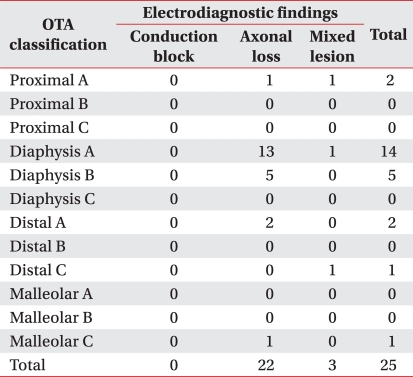
Among all subjects, 9 patients had fibular head fracture whereas 25 patients did not. According to OTA classification, 25 patients without fibular head fracture were classified again. Diaphyseal segment fracture were the most common (19 patients) and proximal segment fracture were 2 patients, distal segment fractures were 3 patients, malleolar segment fracture was 1 patients respectively.
In minor classification, 19 patients with diphyseal segment fracture consisted of 14 type A patients, 5 type B patients and there was no type C patient in diaphyseal segment fracture. Proximal segment fracture consisted only of 2 type A patients, distal segment fracture consisted of 2 type A patients and 1 type C patient and malleolar segment fracture consisted of 1 type C patient (Table 2).
The total 34 patients consisted of 27 patients with common peroneal neuropathy and 7 patients with deep peroneal neuropathy. According to the severity in electrodiagnostic findings, incomplete lesions were in 32 patients, which greatly outnumbered the complete lesions (2 patients). Axonal loss lesions were the most common (30 patients), mixed axonal loss/conduction block lesions were 4 patients and there was no conduction block lesion in our study by classification suggested by Katirji and Willbourn.2
Nine patients with fibular head fracture were all diagnosed as common peroneal neuropathy and 25 patients without fibular head fracture consisted of 18 patients with common peroneal neuropathy and 7 patients with deep peroneal neuropathy in electrodiagnosis (Table 3).
In comparison of the severity between two groups classified according to the existence of fibular head fracture, the group with fibular head fracture consisted of 1 patient with complete lesion and 8 patients with incomplete lesion, and the group without fibular head fracture consisted of 1 patient with complete lesion and 24 patients with incomplete lesion. There was no statistical significance in correlation between the existence of fibular head fracture and the severity of peroneal neuropathy (p=0.437, Fig. 2).
In comparison of the electrodiagnostic findings between 2 groups, group with fibular head fracture consisted of 8 patients with axonal loss lesion and 1 patient with mixed axonal loss/conduction block lesion, and group without fibular head fracture consisted of 22 patients with axonal loss lesion and 3 patients with mixed axonal loss/conduction block lesion. There was no statistical significance in correlation between the existence of fibular head fracture and the electrodiagnostic findings (p=0.723, Fig. 3).
A total of 25 patients without fibular head fracture were classified into groups according to the location of the fracture and the difference of each group in the electrodiagnostic findings, and the severity was evaluated. Proximal segment fracture consisted of 2 patients with incomplete lesion. Diaphyseal segment fracture group was 19 patients and consisted of 18 patients with incomplete lesion and 1 patient with complete lesion. Distal segment fracture group consisted of 3 patients with incomplete lesion, and malleolar segment fracture group consisted of 1 patient with incomplete lesion. There was no statistical significance in correlation between the location of fracture and the severity of peroneal neuropathy in the electrodiagnostic findings (p=0.954, Fig. 4).
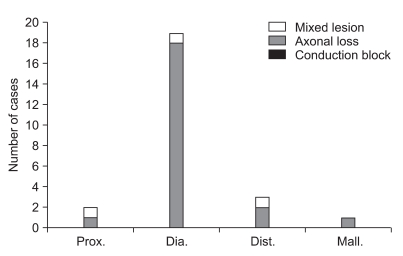
According to the classification suggested by Katirji and Willbourn, 2 patients with proximal segment fracture consisted of 1 patient with axonal loss lesion and 1 patient with mixed axonal loss/conduction block lesion. Nineteen patients of the diaphyseal segment fracture consisted of 17 patients with axonal loss lesion and 2 patients with mixed axonal loss/conduction block lesion. Three patients with distal segment fracture consisted of 2 patients with axonal loss lesion and 1 patient with mixed axonal loss/conduction block lesion and 1 patient with malleolar segment fracture was identified as axonal loss lesion. There was no statistical significance in correlation between the electrodiagnostic findings and the location of the fracture (p=3.91, Fig. 5).
There were only 9 patients out of the total 34 with fibular head fracture that were considered to have inflicted direct damage to the peroneal nerve in this study. On the other hand, there were 25 patients without fibular head fracture, which outnumbered the patients with fibular head fracture. The electrodiagnostic findings of 25 patients without fibular head fracture were investigated and there were 18 patients with peroneal neuropathy and 7 patients with deep peroneal neuropathy. Common peroneal nerve, as a lateral branch of sciatic nerve, is separated from a sciatic nerve at the distal third portion of a thigh, and descends posterior to the fibular head, winding around the neck of the fibula, running toward the anterior portion of the leg, then enters the anterior compartment penetrating through the superficial head of the peroneus longus muscle. The fiber of peroneus longus muscle at this part comprises the tendinous arch called fibular tunnel right above the common peroneal nerve, which is known as one of the causes of peroneal neuropathy.3 After passing through the fibular tunnel, the common peroneal nerve divides in to the deep peroneal nerve and superficial peroneal nerve.
Considering the course of peroneal nerve and the anatomy around fibular head, all patients without fibular head fracture should have been diagnosed as deep peroneal neuropathy. However, 18 out of 25 patients were diagnosed as common peroneal neuropathy.
This study showed that among patients with peroneal neuropathy after traumatic tibio-fibular fracture, there were more patients without fibular head fracture that would have been thought to inflict direct damage on the peroneal nerve than patients with fibular head fracture, and there were more patients diagnosed as common peroneal neuropathy than patients diagnosed as deep peroneal neuropathy by electrodiagnosis. This study also showed that diaphyseal segment fracture was the most common type of tibio-fibular fracture among patients without fibular head fracture who complained of foot drop, and type A (simple type) was the most common type (14 cases) among the diaphyseal segment fracture.
These results implied that peroneal neuropathy after tibio-fibular fracture was caused more frequently by indirect injury such as traction injury, compression over the fibular head after surgery or immobilization, compartment syndrome or entrapment neuropathy than by direct injury such as direct compression or dissection. In other words, direct nerve injury was not the only reason that caused peroneal neuropathy.
Possible mechanisms for indirect injury of peroneal nerve are as follows. First, the limited motion toward the longitudinal direction of common peroneal nerve can be a cause of indirect injury. Berry and Richardson3 reported that fibular tunnel limited the motion toward the longitudinal direction of common and deep peroneal nerves 0.5 cm at most and due to this, severe ankle inversion or posterior dislocation of hip joint could cause the traction force over common peroneal nerve toward the longitudinal direction.16-18
Second, the fact that the traction injury can occur over the entire length of nerve as well as the maximal point of the traction supports the common peroneal neuropathy that occurs after the traction that inflict maximal damage on deep peroneal nerve, distal portion of common peroneal nerve. Haftek19 reported the result that nerve fibers and endoneurial tubes were damaged at the point even except the maximal point of the damage.
Third, peroneal nerve can be damaged by the swelling or hemorrhage inside the nerve at the fibular tunnel. Nobel20 reported 2 cases of peroneal neuropathy caused by hematoma inside the peroneal nerve sheath after diaphyseal fracture of tibia and suggested the mechanism the torsional forces that occurred during the trauma transmitted the traction force along the nerve and dissected the nutritional vessels of the nerve, consequently inflicting the nerve damage. Stoff and Greene18 suggested the hypotheses acute onset peroneal neuropathy after ankle inversion was caused by the traction force that inflicted directly on fibular head and delayed onset peroneal neuropathy after ankle inversion was caused by the hematoma within the peroneal nerve. The subjects had tibio-fibular fracture without ankle inversion in this study. However, an external force strong enough to cause the fracture could outweigh the traction force that occurred at ankle inversion. Therefore, the above two mechanisms can be applied to our subjects. Also, Whitesides and Heckman21 reported neuropathy caused by the compartment syndrome occurred in 10% of patients with closed fracture of tibia.
Fourth, compression caused by immobilization such as splint or cast and leg edema as a cause of peroneal neuropathy during treatment process can be a cause of indirect injury. Baima and Krivickas22 reported that injury of ligament or bone could cause peroneal neuropathy, but peroneal neuropathy occurred more frequently during treatment process than during traumatic event and mentioned these. There was a general notion that nerve injury after fracture would be caused by direct trauma, but as mentioned above, there are many cases that have reported consistently about peroneal neuropathy that occurred during the treatment or healing process of fracture. In addition, there are many cases that have been reported about other peripheral neuropathies that occurred during the healing or treatment process of fracture.23,24
Meanwhile, the fact that common peroneal neuropathy was covered only by the skin and fascia at the head and neck portion of the fibula support the hypothesis that peroneal nerve can be easily damaged by the direct contusion or laceration. In other words, peroneal nerve can be damaged by the direct injury mechanism. Nine cases in our study had fibular head fracture, and the direct damage caused by contusion or compression could be suspected as the mechanism of peroneal nerve injury.
In this study, there was no statistical significance in the correlation between the existence of fibular head fracture and the severity or the electrodiagnostic findings. Neither was there any statistical significant relationship between the site of fracture and the severity or the electrodiagnostic findings in the group without fibular head fracture. Therefore we expect there would be no difference in prognosis according to the existence of fibular head fracture or the site of fracture. Also, considering the fact that there were only 2 patients with complete lesion out of the total set of 34 patients, close observation for occurrence of peroneal neuropathy during healing and treatment process will be needed and if peroneal neuropathy occurs, intensive rehabilitation and follow up of patients will be needed because there is a possibility for recovery.
In our study, there were 9 patients that could be considered as cases for direct nerve injury, even though there were more patients that could be considered as patients with the indirect nerve injury, peroneal neuropathy by the direct nerve injury must not be underestimated.
There are some limitations in our study. The number of subjects was relatively small. Electrodiagnostic studies were not performed by one person, because this study was performed retrospectively, we could not consider the error of inter-reliability. In addition, we could not fully estimate the relationship between the electrodiagnostic study and clinical symptoms because of poor description of medical records and it was not easy for us to check correct duration from the onset in some cases. Imaging studies such as ultrasonography were not performed in the group that was considered as the indirect nerve injury to find out structural damage of the nerve. However, it was meaningful to verify that there was no statistical significance in the correlation between the severity and incidence of peroneal neuropathy or the site of fracture. It would be beneficial in further research for mechanism of peroneal neuropathy, to perform combined imaging studies such as ultrasonography or MRI for confirmation of the structural damage and study the relation between those findings with the electrodiagnostic findings.
There were more cases without fibular fracture than cases with fibular head fracture which were considered as direct nerve injury cases in patients with peroneal neuropathy after tibio-fibular fracture. In addition, among patients without fibular head fracture, cases diagnosed as common peroneal neuropathy outnumbered the cases diagnosed as deep peroneal neuropathy. These results suggested that peroneal neuropathy occurred not only by direct nerve injury but also by indirect nerve injury. We must pay attention to the patients with tibio-fibular fracture that do not involve the fibular head as well as the patients with fibular head fracture who are vulnerable to peroneal nerve injury in anatomical point of view. Considering the facts that there was no statistical significance between the site of tibio-fibular fracture and the severity of peroneal neuropathy and incomplete lesions outnumbered complete lesions, more favorable prognosis can be expected, therefore intensive rehabilitation will be needed.
References
1. Clawson DK, Seddon HJ. The late consequences of sciatic nerve injury. J Bone Joint Surg Br. 1960; 42:213–225. PMID: 13849404.

2. Katirji MB, Wilbourn AJ. Common peroneal mononeuropathy: a clinical and electrophysiologic study of 116 lesions. Neurology. 1988; 38:1723–1728. PMID: 2847078.

3. Berry H, Richardson PM. Common peroneal nerve palsy: a clinical and electrophysiological review. J Neurol Neurosurg Psychiatry. 1976; 39:1162–1171. PMID: 1011026.

4. Smith T, Trojaborg W. Clinical and electrophysiological recovery from peroneal palsy. Acta Neurol Scand. 1986; 74:328–335. PMID: 3811839.

5. Oh SJ, Demirci M, Dajani B, Melo AC, Claussen GC. Distal sensory nerve conduction of the superficial peroneal nerve: new method and its clinical application. Muscle Nerve. 2001; 24:689–694. PMID: 11317280.

6. Marciniak C, Armon C, Wilson J, Miller R. Practice parameter: utility of electrodiagnostic techniques in evaluating patients with suspected peroneal neuropathy: an evidence-based review. Muscle Nerve. 2005; 31:520–527. PMID: 15768387.

7. Ayyar R. MR imaging of the lower leg versus clinical electrophysiologic examination in the differential diagnosis of neurogenic foot drop. AJNR Am J Neuroradiol. 2003; 24:1273–1274. PMID: 12917110.
8. Singh H, Behse F, Buchthal F. Electrophysiological study of peroneal palsy. J Neurol Neurosurg Psychiatry. 1974; 37:1202–1213. PMID: 4376162.

9. Masakado Y, Kawakami M, Suzuki K, Abe L, Ota T, Kimura A. Clinical neurophysiology in the diagnosis of peroneal nerve palsy. Keio J Med. 2008; 57:84–89. PMID: 18677088.

10. Kiechl S. Peer S, Bodner G, editors. Clinical and Electrodiagnostic Work-Up of Peripheral Nerve Lesions. High resolution sonography of the peripheral nervous system. 2008. 2nd ed. Berlin: Springer;p. 52–53.

11. Dumitru D, Amato AA, Zwarts MJ. Dumitru D, Amato AA, Zwarts MJ, editors. Peripheral nervous system's reaction to injury. Electrodiagnostic medicine. 2002. 2nd ed. Philadelphia: Hanley & Belfus;p. 129.

12. Dumitru D, Amato AA, Zwarts MJ. Dumitru D, Amato AA, Zwarts MJ, editors. Nerve conduction studies. Electrodiagnostic medicine. 2002. 2nd ed. Philadelphia: Hanley & Belfus;p. 159–223.

13. Kim DH, Yim SK, Kwon HK, Lee HJ. Clinical and electrophysiologic review of patients with common peroneal mononeuropathy. J Korean Assoc EMG-Electrodiagn Med. 2000; 2:17–23.
15. Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, Prokuski L, Sirkin MS, Ziran B, Henley B, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. J Orthop Trauma. 2007; 21:S1–S133. PMID: 18277234.
16. Meals RA. Peroneal-nerve palsy complicating ankle sprain. Report of two cases and review of the literature. J Bone Joint Surg Am. 1977; 59:966–968. PMID: 409719.

17. Johnston EC, Howell SJ. Tension neuropathy of the superficial peroneal nerve: associated conditions and results of release. Foot Ankle Int. 1999; 20:576–582. PMID: 10509685.

18. Stoff MD, Greene AF. Common peroneal nerve palsy following inversion ankle injury: a report of two cases. Phys Ther. 1982; 62:1463–1464. PMID: 7122703.
19. Haftek J. Stretch injury of peripheral nerve. Acute effects of stretching on rabbit nerve. J Bone Joint Surg Br. 1970; 52:354–365. PMID: 5445417.
20. Nobel W. Peroneal palsy due to hematoma in the common peroneal nerve sheath after distal torsional fractures and inversion ankle sprains. J Bone Joint Surg Am. 1966; 48:1484–1495. PMID: 4289139.

21. Whitesides TE, Heckman MM. Acute Compartment Syndrome: Update on Diagnosis and Treatment. J Am Acad Orthop Surg. 1996; 4:209–218. PMID: 10795056.

22. Baima J, Krivickas L. Evaluation and treatment of peroneal neuropathy. Curr Rev Musculoskelet Med. 2008; 1:147–153. PMID: 19468889.

23. Vural M, Arslantas A. Delayed radial nerve palsy due to entrapment of the nerve in the callus of a distal third humerus fracture. Turk Neurosurg. 2008; 18:194–196. PMID: 18597237.
24. Bodner G, Buchberger W, Schocke M, Bale R, Huber B, Harpf C, Gassner E, Jaschke W. Radial nerve palsy associated with humeral shaft fracture: evaluation with US--initial experience. Radiology. 2001; 219:811–816. PMID: 11376275.

Fig. 1
OTA (Orthopedic Trauma Association) classification of tibio-fibular fracture. Tibio-fibular fracture is classified as proximal, diaphyseal, distal and malleolar segment according to the anatomical site of the fracture.
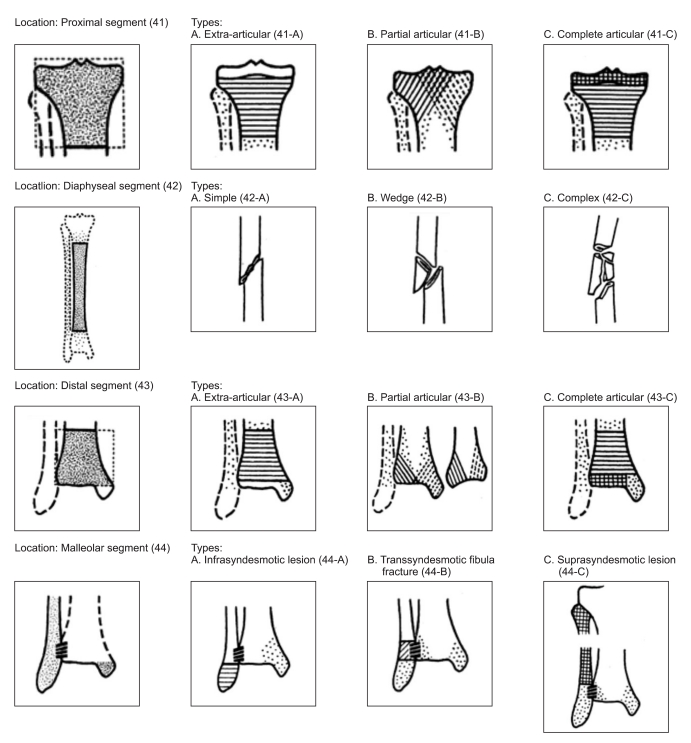
Fig. 2
Distribution of severity according to the injury mechanism. There was one complete lesion and 8 incomplete lesions in groups with fibular head fracture, and there was one complete lesion and 24 incomplete lesions in groups without fibular head fracture. There was no statistical significance in the relationship between the injury mechanism and severity.

Fig. 3
Distribution of electrodiagnostic findings according to the injury mechanism. There was one mixed lesion and 8 axonal loss lesions in groups with fibular head fracture, and there were 3 mixed lesions and 22 axonal loss lesions in groups without fibular head fracture. There was no statistical significance in the relationship between the injury mechanism and the electrodiagnostic findings.
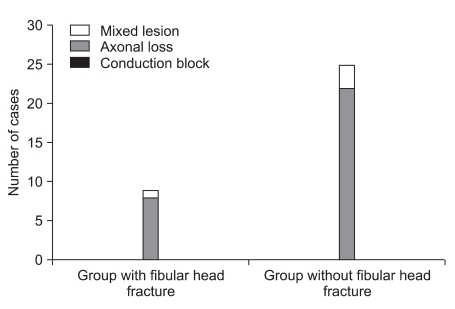
Fig. 4
Distribution of severity in the electrodiagnostic findings according to fracture patterns in patients without fracture around the fibular head (Prox.: proximal segment fracture, Dia.: diaphyseal segment fracture, Dist.: distal segment fracture, Mall.: malleolar segment fracture). 1 complete lesion was found in diaphyseal segment fracture and 24 incomplete lesions were found, 2 cases in proximal segment fracture, 18 cases in diaphyseal segment fracture, 3 cases in distal segment fracture, 1 case in malleolar segment fracture. There was no statistical significance in the relationship between the anatomical site of fracture and the severity.
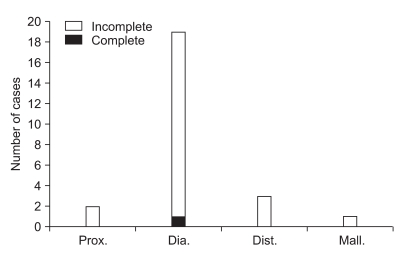
Fig. 5
Distribution of electrodiagnostic findings according to fracture patterns in patients without fibular head fracture (Prox.: proximal segment fracture, Dia.: diaphyseal segment fracture, Dist.: distal segment fracture, Mall.: malleolar segment fracture). 4 mixed lesions were found, 1 case in proximal segment fracture, 2 cases in diaphyseasl segment fracture and 1 case in distal segment fracture. 21 axonal loss lesions were found, 1 case in proximal segment fracture, 17 cases in diaphyesal segment fracture, 2 cases in distal segment fracture and 1 case in malleolar segment fracture. There was no statistical significance in the relationship between the anatomical site of fracture and the electrodiagnostic findings.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download