Abstract
Objective
To evaluate the spasticity and electrophysiologic effects of applying extracorporeal shock wave therapy (ESWT) to the gastrocnemius by studying F wave and H-reflex.
Method
Ten healthy adults and 10 hemiplegic stroke patients with ankle plantarflexor spasticity received one session of ESWT on the medial head of the gastrocnemius. The modified Ashworth scale (MAS), tibial nerve conduction, F wave, and H-reflex results were measured before and immediately after the treatment. The Visual Analogue Scale (VAS) was used during ESWT to measure the side effects, such as pain.
Results
There were no significant effects of ESWT on the conduction velocity, distal latency and amplitude of tibial nerve conduction, minimal latency of tibial nerve F wave, latency, or H-M ratio of H-reflex in either the healthy or stroke group. However, the MAS of plantarflexor was significantly reduced from 2.67±1.15 to 1.22±1.03 (p<0.05) after applying ESWT in the stroke group.
Spasticity is one of the most important causes of disability in stroke patients; it interferes with the patients' rehabilitation, especially regarding functional recovery.1 Because the primary lesion causing spasticity is located in the central nervous system, most studies have focused on the neural mechanisms such as excessively increased spinal excitability as the primary cause of spasticity.2,3 In recent years, there have been several reports that chronic spasticity itself plays a role in the increase of joint resistance by structural and mechanical changes in skeletal muscles through fibrosis of connective tissue.4
Recent studies have reported that intramuscular injections of botulinum toxin type A reduces spasticity in hypertonic muscles of the upper limb or equinovarus deformity by lower limb spasticity, and improves functional abilities.5-7 However, the development of neutralizing antibodies can reduce the efficacy of treatment for some patients. In addition, the dosage of botulinum toxin is not always sufficient to treat extensive and severe hypertonia in the upper and lower limbs. Chronic hypertonic muscles can lead to the fibrosis of connective tissue, and should be considered in the rehabilitation of stiff joints.8
Extracorporeal shock wave therapy (ESWT), defined as a sequence of single sonic pulses characterized by high peak pressure (100 MPa), fast pressure rise (<10 ns), and short duration (10 µs), is conveyed by an appropriate generator to a specific target area with an energy density in the range of 0.003-0.890 mJ/mm2. ESWT was first applied to patients in 1980 to break up kidney stones.9 Recent studies have indicated that ESWT is effective for treatment of musculoskeletal diseases such as pseudoarthrosis, calcific tendinitis of shoulder, epicondylitis, plantar fasciitis, and several inflammatory tendon diseases.10-13 Mariotto et al.14 reported that ESWT induced nitric oxide (NO) synthesis, and NO plays a critical role in anti-inflammatory responses.
The amplitude of the F wave and the F-M ratio reflect the excitability of motor neurons and are increased by spasticity.15-17 Many studies have reported that the amplitude of the F wave and the F-M ratio can be used as tools for measuring the spasticity electrophysiologically. Latency of H-reflex and H-M ratio, which reflect the excitability of α motor neurons, can also be used as a tool for measuringspasticity.18,19
Manganotti and Amelio20 reported that ESWT on the flexor hypertonic muscles of the forearm and the interosseus muscles of the hand was effective for the improvement of upper limb spasticity in stroke patients for >12 weeks; 1,500 shots were used to treat flexor muscles of the forearm and 800 shots for each interosseus muscle of the hand with 0.030 mJ/mm2 intensity. Yoo et al.21 reported significantly reduced spasticity on the elbow flexor and wrist pronator for 1 to 4 weeks after 1,000 shots of ESWT with 0.069 mJ/mm2 intensity; however, there was no report on ESWT's therapeutic effect on lower limb spasticity for stroke patients, and it's mechanism is still unknown. The goal of this study was to evaluate the spasticity and electrophysiologic effects by studying F wave and H-reflex after applying ESWT on the gastrocnemius in stroke patients with lower limb spasticity.
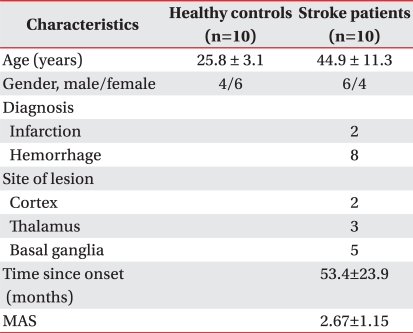
Ten healthy adults (4 males and 6 females, mean age of 25.8±3.1 years) and 10 hemiplegic stroke patients with ankle plantarflexor spasticity (6 males and 4 females, mean age of 44.9±11.3 years) were enrolled for this study. Two patients had ischemic stroke, and 8 patients had hemorrhagic stroke. Two patients had cortical lesions, 3 patients had thalamic lesions, and 5 patients had basal ganglia lesions. All patients had a spasticity greater than grade I, by modified Ashworth scale (MAS) and were in a chronic stage for over 15 months. Patients with previous or planned treatment of the limb with botulinum toxin, phenol, or intrathecal baclofen pump were excluded. All patients were receiving antispastic pharmacological therapy and did not change the dose of antispastic medication during the study (Table 1).
Evotron® (SwiTech, Kreuzlingen, Switzerland) was used for ESWT. The pressure pulses were focused at the medial head of the gastrocnemius of the spastic side. Shots (1,500) were given in the middle of the belly and the intensity was 0.1 mJ/mm2. In the healthy control group, the same methods of ESWT were applied at the right side medial head of the gastrocnemius. R20 with the focusing depth of 20-30 mm was used for the head of ESWT to minimize or avoid stimulation of the soleus.
F wave and H-reflexes of the tibial nerve were recorded before and immediately after the treatment to assess the electrophysiologic effects of ESWT on spasticity. Medelec Synergy® (Viasys healthcare, San Diego, USA) was used for electrophysiologic assessment and all studies were performed by one rehabilitation physiatrist. In the F wave study, an active recording electrode was placed in the abductor hallucis muscle and the reference electrode was at the proximal interphalangeal joint of the great toe. Supramaximal stimulation of the tibial nerve was done 20 times on the posterior area of the ankle medial malleolus using antidromic conduction, and the F wave's minimal latency was recorded. The sweep speed was 5-10 ms/div, sensitivity was 200-500 µV/div, and the filter range was 20-10,000 Hz. In the H-reflex study, the active recording electrode was placed in the soleus muscle and the reference electrode in the Achilles tendon. To evoke H-reflex, the posterior tibial nerve was stimulated in popliteal fossa with 0.2 Hz frequency and 0.5 ms duration. We gradually increased stimulation intensity and recorded H-reflex latency and H-M ratio using the maximal amplitude of H-reflex and M wave. Distal latency, amplitude of compound muscle action potential, and conduction velocity of the tibial nerve were recorded to assess the peripheral nerve injury by ESWT.
Modified Ashworth Scale (MAS) was used to assess the spasticity of an ankle plantarflexor. MAS is a velocity-independent method which manually assesses the joint range of motion. For convenience of statistical analysis, MAS grade 1+ was matched to point 2; grades 2, 3 and 4 were matched to 3, 4 and 5, respectively.22 To avoid inter-rater bias, all studies were performed by one rehabilitation physiatrist.
The Visual Analogue Scale (VAS) was used to estimate the severity of pain produced by ESWT. A vertical line with 10 cm length on paper was presented to the subjects and the subjects were told that the lower end of the line indicated "pain-free state" and the upper end indicated "can not endure because of too severe pain." After ESWT, all subjects were drawing the perpendicular line on the vertical line based on their pain experienced during treatment. Vertical lines were divided by 10 grades with 1 cm intervals, and this indicated the degree of pain.23
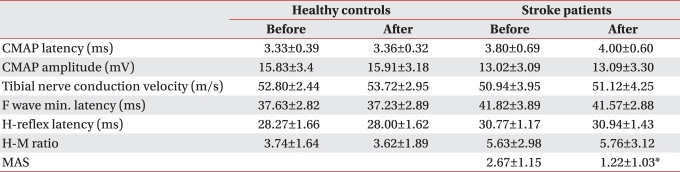
No significant changes were found in the F wave minimal latency, H-reflex latency or H-M ratio after ESWT in healthy controls. Likewise, changes in the latency, amplitude of compound muscle action potential and conduction velocity of the tibial nerve were not significant (Table 2). Mild pain was experienced during ESWT, VAS 2.87±1.49.
In stroke patients, MAS of ankle plantarflexor before treatment was 2.67±1.15, and was significantly reduced to 1.22±1.03 immediately after ESWT (p<0.05) (Table 2). Also, mild pain (VAS 3.23±1.28) was produced by ESWT.
In stroke patients, F wave minimal latency, H-reflex latency, and H-M ratio before treatment were 41.82±3.89 ms, 30.77±1.17 ms, and 5.63±2.98, respectively. Immediately after treatment, they were 41.57±2.88 ms, 30.94±1.44 ms, and 5.76±3.12 ms, respectively. There were no significant changes after treatment (Table 2).
In stroke patients, the latency and amplitude of tibial nerve compound muscle action potential before treatment were each 3.80±0.69 ms and 13.02±3.10 mA. Immediately after treatment, these values were 4.00±0.60 ms and 13.09±3.30 mA, respectively, and no significant changes were noted after treatment (Table 2). Also, there was no significant change of tibial nerve conduction velocity from 50.94±3.95 m/s before treatment to 51.12±4.25 m/s after treatment (Table 2).
ESWT is known to be effective in musculoskeletal diseases for treating pain, inflammation, or injury of a ligament. ESWT produces mechanical effects such as regeneration of degenerated tissue, neovascularization, and resorption of calcium deposit,24,25 and also physiological responses such as changes of epithelial cell permeability, free radical formation, change of cell membrane permeability, NO formation, and variable growth factor formation.14,20,26
The mechanisms of ESWT on spasticity due to central nervous system injury are still unknown. Mariotto et al.14 stated that ESWT can induce NO synthesis and NO is involved in improvement of variable tendon disease. NO is involved in neuromuscular junction formation in the peripheral nervous system27 and in physiological functions of the central nervous system, including neurotransmission, and synaptic plasticity.28
Likewise, variable mechanisms have been proposed, including acting on fibrosis of chronic hypertonic muscles,20 decreasing spinal excitability,29 and mechanical vibratory stimulation.29 Considering the therapeutic effects of ESWT on bones and tendons Manganotti and Amelio20 proposed that reduction of spasticity could be caused by improving the stiffness of connective tissue by directly acting on fibrosis of chronic hypertonic muscles.
In this study, we attempted to evaluate the antispastic mechanism of ESWT by using F waves and H-reflexes that reflect spinal excitability. Results showed no significant changes in F wave minimal latency, H-reflex latency, or H-M ratio after treatment. Therefore, those factors can be eliminated as the mechanism by which ESWT reduces spasticity by decreasing spinal excitability. Manganotti and Amelio20 also reported that the effect of ESWT on spinal excitability can be excluded as the main mechanism, because no change of F wave latency or amplitude after treatment was demonstrated.
Another possible mechanism was mechanical vibratory stimulation, which reduces excitability of motor neurons and induces the change of F wave.29 In this study, we can rule out this theory because no significant change of F wave or H-reflex were detected. Considering the clinical anti-spastic effect observed up to 4 weeks after treatment,20 mechanical vibratory stimulation, which is transitory and short lasting, could also be excluded as a major effect.
Because the latency and amplitude of compound muscle action potential and conduction velocity of tibial nerve were not changed in this study, we can rule out a significant effect of ESWT on peripheral nerves and the effect of botulinum toxin A on neuromuscular blockage.
Results of this study could not reveal the exact mechanism of ESWT for reducing spasticity. However, we can rule out several proposed mechanisms such as decreased spinal excitability, peripheral nerve injury, and mechanical vibratory stimulation by using electrophysiologic studies. It was previously reported that range of motion of the joint increased after ESWT;20,21 however, we did not evaluate range of motion of the joint in the study, and therefore the mechanisms proposed by Maganotti and Amelio20 for reducing spasticity mediated by improving stiffness of connective tissue affecting fibrosis of chronic hypertonic muscles were not confirmed in this study.
Similar to previous studies performed in stroke patients with upper limb spasticity, an immediate therapeutic effect of ESWT in reducing spasticity was found for stroke patients with ankle spasticity. ESWT can reduce spasticity in stroke patients with lower limb spasticity and prevent complications of spasticity, such as equinovarus deformity. This study only measured the immediate effect of ESWT, but Manganotti and Amelio20 and Yoo et al.21 reported that the therapeutic effect of ESWT on upper limb spasticity could last at least 4 weeks, maximally 12 weeks, and that ESWT had a therapeutic effect on reducing spasticity for a considerable time. There were several cases where the patient complained of pain during treatment, but the degree of pain was not severe (< VAS 3), and not all subjects reported pain after treatment; other side effects were not found in this study. ESWT appears to be safe and noninvasive and can reduce financial burden compared to treatment using botulinum toxin A. ESWT can be helpful in treatment of chronic stroke patients with spasticity.
There are several limitations in this study. First, the mechanism of ESWT for reducing spasticity was not fully evaluated in this study. Further studies are needed to elucidate the mechanism of ESWT for reducing spasticity by comparing the anti-spastic effects; for example, stiffness of connective tissue for acute stroke patients compared with chronic stroke patients. Second, this study used F wave minimal latency to assess the spinal excitability instead of F wave amplitude or F-M ratio, which are well known and widely used. Further studies concerning the correlation between F wave minimal latency and spinal excitability are needed. Third, similar to results from previous studies conducted for upper limb spasticity,20,21 range of motion of the joint should be measured. Reduced effect of spasticity on functional abilities such as ambulation or activities of daily living should be assessed. Finally, further studies concerning the most effective level of intensity, number of ESWT treatments, and duration of therapeutic effect, need to be conducted in a larger number of patients.
In this study, 1 session of ESWT for chronic stroke patients with ankle plantarflexor spasticity produced an immediate effect. There were no significant changes in electrophysiologic studies such as F wave, H-reflex, or tibial nerve conduction studies after treatment. ESWT appears to be safe and noninvasive, and can reduce financial burden compared to treatment with botulinum toxin A. ESWT can be helpful for treatment of stroke patients with spasticity, prevention of complications such as equinovarus deformity, and result in a more stable gait pattern. Further studies in larger numbers of patients on functional changes produced by reduced spasticity, the most effective treatment protocol for ESWT, and duration of therapeutic effect, are warranted.
References
1. Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994; 75:394–398. PMID: 8172497.

2. Duncan PW, Wallace D, Studenski S, Lai S, Johnson D. Conceptualization of a new stroke-specific outcome measure: the stroke impact scale. Top Stroke Rehabil. 2001; 8:19–33. PMID: 14523743.

3. Sehgal N, McGuire JR. Beyond Ashworth. Electrophysiologic quantification of spasticity. Phys Med Rehabil Clin N Am. 1998; 9:949–979. PMID: 9894105.

4. Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil. 1989; 70:144–155. PMID: 2644919.
5. Cardoso E, Pedreira G, Prazeres A, Ribeiro N, Melo A. Does botulinum toxin improve the function of the patient with spasticity after stroke. Arq Neuropsiquiatr. 2007; 65:592–595. PMID: 17876396.

6. Barwood S, Baillieu C, Boyd R, Brereton K, Low J, Nattrass G, Graham HK. Analgesic effects of botulinum toxin A: a randomized, placebo-controlled clinical trial. Dev Med Child Neurol. 2000; 42:116–121. PMID: 10698329.

7. Rousseaux M, Kozlowski O, Froger J. Efficacy of botulinum toxin A in upper limb function of hemiplegic patients. J Neurol. 2002; 249:76–84. PMID: 11954872.

8. Lagalla G, Danni M, Reiter F, Ceravolo MG, Provinciali L. Post-stroke spasticity management with repeated botulinum toxin injections in the upper limb. Am J Phys Med Rehabil. 2000; 79:377–384. PMID: 10892624.

9. Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stone by shock waves. Lancet. 1980; 2:1265–1268. PMID: 6108446.
10. Valchanou VD, Michailov P. High energy shock waves in the treatment of delayed and nonunion of fractures. Int Orthop. 1991; 15:181–184. PMID: 1743828.

11. Rompe JD, Zoellner J, Nafe B. Shock wave therapy versus conventional surgery in the treatment of calcifying tendinitis of the shoulder. Clin Orthop Relat Res. 2001; 387:72–82. PMID: 11400897.

12. Rompe JD, Hopf C, Kullmer K, Heine J, Burger R. Analgesic effect of extracorporeal shock-wave therapy on chronic tennis elbow. J Bone Joint Surg Br. 1996; 78:233–237. PMID: 8666632.

13. Rompe JD, Decking J, Schoellner C, Nafe B. Shock wave application for chronic plantar fasciitis in running athletes. A prospective, randomized, placebocontrolled trial. Am J Sports Med. 2003; 31:268–275. PMID: 12642264.

14. Mariotto S, Cavalieri E, Amelio E, Ciampa AR, de Prati AC, Marlinghaus E, Russo S, Suzuki H. Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide. 2005; 12:89–96. PMID: 15740982.

15. Kimura J. Electrodiagnosis in diseases of nerve and muscle: principles and practice. 2001. UK: University of Oxford;p. 439–465.
16. Eisen A, Odusote K. Amplitude of the F-wave: a potential means of documenting spasticity. Neurology. 1979; 29:1306–1309. PMID: 573413.
17. Milanov I. Examination of the segmental pathophysiological mechanisms of spasticity. Electromyogr Clin Neurophysiol. 1994; 34:73–79. PMID: 8187681.
18. Hultborn H, Nielsen J. H-reflexes and F-responses are not equally sensitive to changes in motoneuronal excitability. Muscle Nerve. 1995; 18:1471–1474. PMID: 7477072.

19. Bakheit AM, Maynard VA, Curnow J, Hudson N, Kodapala S. The relationship between Ashworth scale scores and the excitability of the alpha motor neurones in patients with post-stroke muscle spasticity. J Neurol Neurosurg Psychiatry. 2003; 74:646–648. PMID: 12700310.
20. Manganotti P, Amelio E. Long-term effect of shock wave therapy on upper limb hypertonia in patients affected by stroke. Stroke. 2005; 36:1967–1971. PMID: 16109905.

21. Yoo SD, Kim HS, Jung PK. The effect of shock wave therapy on upper limb spasticity in the patients with stroke. J Korean Acad Rehabil Med. 2008; 32:406–410.
22. Han TR, Kim JH, Chun MH. Evaluation of spasticity in hemiplegic patients. J Korean Acad Rehabil Med. 1993; 17:18–25.
23. McDowell I, Newell C. Measuring health; a guide to rating scales and questionnaires. 1987. UK: University of Oxford;p. 229–268.
24. Wang CJ, Huang HY, Pai CH. Shock wave-enhanced neovascularization at the tendon-bone junction: an experiment in dogs. J Foot Ankle Surg. 2002; 41:16–22. PMID: 11858601.

25. Wang CJ, Wang FS, Yang KD, Weng LH, Hsu CC, Huang CS, Yang LC. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in the rabbits. J Orthop Res. 2003; 21:984–999. PMID: 14554209.
26. Seidl M, Steinbach P, Worle K, Hofstadter F. Induction of stress fibres and intercellular gaps in human vascular endothelium by shock-waves. Ultrasonics. 1994; 32:397–400. PMID: 8079400.

27. Molina JA, Jimenez-Jimenez FJ, Orti-Pareja M, Navarro JA. The role of nitric oxide in neurodegeneration. Potential for pharmacological intervention. Drugs Aging. 1998; 12:251–259. PMID: 9571390.
28. Blottner D, Luck G. Just in time and place: NOS/NO system assembly in neuromuscular junction formation. Microsc Res Tech. 2001; 55:171–180. PMID: 11747092.

29. Leone JA, Kukulka CG. Effects of tendon pressure on alpha motoneuron excitability in patients with stroke. Phys Ther. 1988; 68:475–480. PMID: 3353457.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download