Abstract
Purpose
The management of patients with colorectal cancer (CRC) who have liver cirrhosis (LC) requires a thorough understanding of both diseases; however, the prognoses and postoperative outcomes of such patients remain understudied. We investigated the effect of LC on surgical and oncologic outcomes in patients with CRC, and identified prognostic factors.
Methods
We analyzed 453 patients with CRC and LC (CRC-LC group), 906 with CRC only (CRC group), 906 with LC only (LC group), and 1,812 healthy subjects using health insurance claim data (2008–2013).
Results
The CRC-LC group had a higher frequency of intensive care unit admission than the CRC group; there were no differences between the 2 groups in terms of early and late postoperative small bowel obstruction and incisional hernia. However, the 30-day, 60-day, and 90-day mortality rates were all significantly higher in the CRC-LC group. The higher Charlson comorbidity index (hazard ratio [HR], 1.127) and the lower socioeconomic status (HR, 0.985) were significant worse predictors of 5-year survival. Patients with underlying LC had a significantly higher HR in both the advanced CRC (HR, 1.858) and nonadvanced CRC (HR, 1.799) subgroups. However, the nonadvanced CRC subgroup showed a lower HR than the LC group (HR, 0.730).
Conclusion
Patients with CRC who had underlying LC had a lower survival rate than did those without LC, although the incidence rates of postoperative complications were not significantly different. The presence of LC was associated with a significantly lower survival rate regardless of CRC presence.
Liver cirrhosis (LC) is a pathological condition where long-term fibrous scarring of the liver owing to persistent inflammation caused by hepatitis virus infection or alcohol consumption leads to the loss of liver function [1]. A deepening pathophysiological understanding of LC and advances in treatment methods, such as liver transplantation, have led to longer life expectancies in patients with LC; however, no notable improvements in postoperative morbidity and mortality rates have been observed [23]. The reported morbidity rates of patients with LC are 30.1%–43% after nonhepatic abdominal surgery, while the reported mortality rates range between 11.1% and 35.8% [456].
Colorectal cancer (CRC) is the fourth most common cancer worldwide accounting for 6.1% of newly diagnosed cancers annually, and is the third leading cause of cancer-related deaths with a mortality rate of 5.8% [7]. Its incidence is rapidly increasing in South Korea owing to the rising popularity of Western diets in recent years [8]. A previous study found evidence of an increased risk of CRC in patients with LC [9]; the management of such patients requires a thorough understanding of the 2 diseases. While CRC and LC have been investigated separately in previous studies, the prognoses and postoperative outcomes of patients with CRC complicated with LC remain understudied. Among patients with CRC, we previously reported a significantly lower 5-year survival rate in those who also had LC (46.7%) than in those who did not (76.2%). We also derived a modified model that includes the serum sodium score that can serve as a prognostic predictor for patients who have both CRC and LC [4]. However, our previous study had a limited sample size; therefore, we performed this study to compare oncological and surgical outcomes between patients with CRC who had LC and those who did not using a nationwide cohort. Furthermore, we examined the respective effects of CRC and LC on the patients' prognoses and surgical outcomes by comparing them to members of the general public who were either healthy or have only LC.
This study was approved by the Institutional Review Board of Dongnam Institute of Radiological and Medical Sciences (No. D-1807-007-002). It was also approved by the National Health Insurance Service (NHIS) Research Committee (NHIS-2018-1-378), and data customized by NHIS researchers were provided.
We performed a nationwide retrospective study using health insurance claim data provided by the NHIS (Republic of Korea), which covers approximately 97% of all medical care and is considered representative of the South Korean population [10]. We included patients who were diagnosed with CRC and underwent resection between 2008 and 2013; there were 105,754 such patients identified. CRC was diagnosed per the Korean Classification of Diseases (KCD) codes, and resection was confirmed according to surgical codes. Patients diagnosed with any other type of cancer within 2 years before the surgery were excluded (n = 39,162). Patients with CRC who underwent resection were also excluded if (1) we were unable to identify their basic demographic characteristics (n = 1,206), (2) the location of CRC was unclear (n = 6,826), or (3) they were younger than 19 years of age at the time of diagnosis (n = 22). Of the remaining 58,538 patients, 453 developed LC within 2 years before surgery; LC was confirmed using the KCD codes. Each patient with both CRC and LC was matched with 2 patients with CRC but without LC according to sex, age (5-year intervals), and year of surgery. Furthermore, CRC patients with and without LC were matched patients without CRC but with LC and patients without CRC and LC, respectively. Upon matching, sex, age (5-year intervals) and the same year were matched, and then patients were randomly extracted at this ratio of 1:2. Therefore, CRC patients with LC, CRC patients without LC, patients without CRC but with LC, and patients without CRC and LC were matched at a ratio of 1:2:2:4. As a result, 453 patients with CRC and LC (the CRC-LC group), 906 with CRC but without LC (CRC group), 906 subjects with LC alone (LC group), and 1,812 healthy patients (control group) were analyzed.
We considered the effects of basic demographic characteristics,
location of CRC, stage of CRC, and surgical method on survival. For basic demographic characteristics, we considered age, sex, and socioeconomic status (SES); the latter was based on the level of insurance premium, which ranged from 0 to 20 (the higher the insurance premium level, the higher the SES). As the survival rates of patients with CRC differ depending on the tumor's location (i.e., the left versus right colon), C185, C186, C187, C19, and C20 codes were classified as left-sided CRCs while C180, C181, C182, C183, and C184 were classified as right-sided. As with all types of cancer, the tumor stage (which is a significant predictor of survival) was determined by whether chemotherapy or radiotherapy were administered; if codes denoting chemotherapy or radiotherapy administration were noted in the patient's medical records 1 year before and after the surgery date, the patient was classified as having advanced-stage CRC. Incisional hernia and ileus were confirmed via KCD and surgical codes. We also considered the surgical method as a covariate; there were 2 types of surgical CRC resection: laparoscopic and open. Lastly, the Charlson comorbidity index (CCI) was included as a covariate, and was calculated by using the CCI calculation algorithm in the analytical manual published by the Health Insurance Review & Assessment Service [11].
The t-test and chi-square test were performed to determine any differences in the means of continuous and categorical variables, respectively, between patients with and without LC. Kaplan-Meier survival curves were plotted for patients with and without LC according to the covariates. The effect of LC on the survival of patients with CRC was tested using a Cox proportional hazards model. For this analysis, the history of CRC and LC, CRC stage, location of CRC, SES, CCI, and surgical method were used as covariates. Sex, age, and year of surgery were not considered covariates because they were used to match patients with and without LC. All statistical analyses were performed using the R statistical software ver. 3.5 (R Foundation for Statistical Computing, Vienna, Austria), and figures were plotted using the Rex software [1213]. P-values of <0.05 were considered statistically significant.
There were 453 patients in the CRC-LC group and 58,085 in the CRC group who underwent surgery for CRC; the characteristics of these patients before and after matching are summarized in Table 1. Compared to the CRC group, the CRC-LC group had a higher percentage of nonadvanced, right-sided CRC and lower SES. Table 2 shows the comparisons of 906 LC-group patient and 1,812 healthy controls.
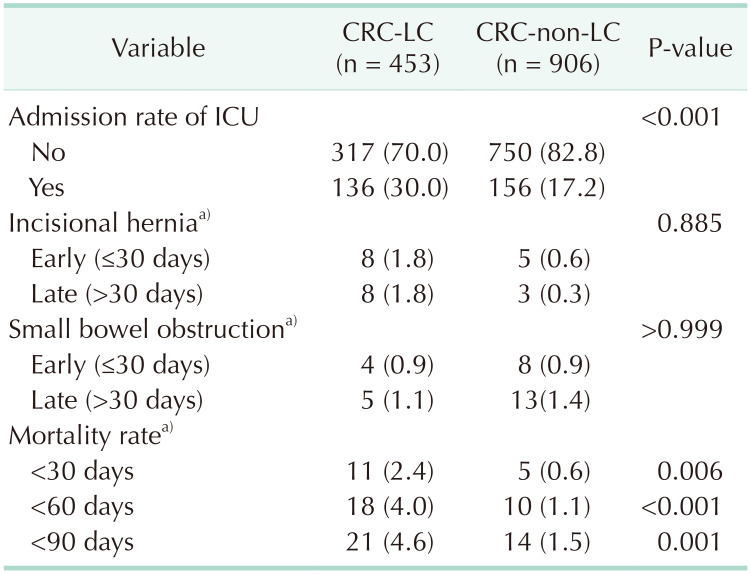
Patients in the CRC-LC group had a higher frequency of intensive care unit (ICU) admission than did those in the CRC group. There were no differences between the 2 groups in the incidences of early (≤30 days) and late (>30 days) postoperative small bowel obstruction and incisional hernia. However, their mortality rates were significantly different, as the 30-day (2.4% vs. 0.6%, P = 0.0058), 60-day (4.0% vs. 1.1%, P < 0.001), and 90-day (4.6% vs. 1.5%, P = 0.0013) mortality rates were all significantly higher in the CRC-LC group than in the CRC group (Table 3).
Patients presenting with underlying LC had a significantly lower survival rate than those without LC. The CRC-LC group had the lowest survival rate (with statistical significance), followed (in order) by the LC, CRC, and control groups. The survival rate of patients with CRC (i.e., the CRC-LC and CRC groups) was significantly lower than that of patients without CRC (the LC and control groups). When patients with CRC were divided into the nonadvanced and advanced disease groups, the control group showed the highest survival rate, followed by the nonadvanced CRC, advanced CRC, LC, nonadvanced CRC-LC, and advanced CRC-LC groups (Fig. 1). In terms of other investigated covariates, a higher CCI, right-sided CRC, SES ≤10, and open surgery were associated with a significantly lower survival rate (Fig. 2).
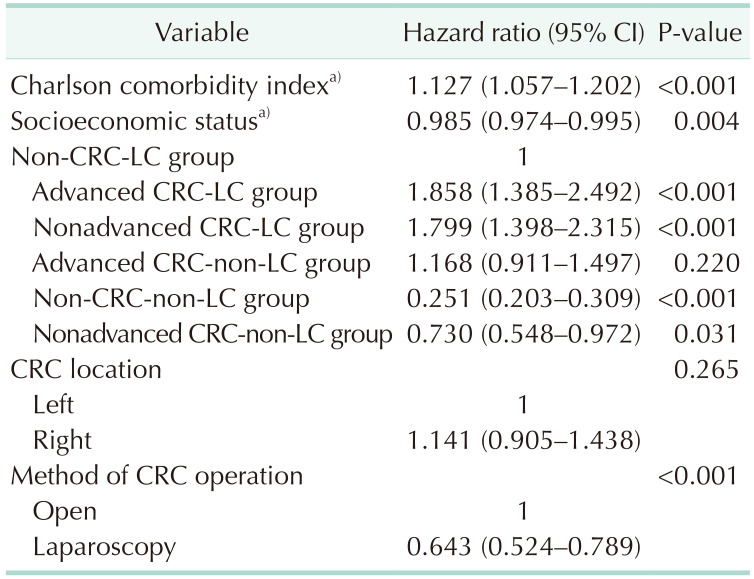
Of the factors influencing the 5-year survival rate, a higher CCI and higher SES were associated with significantly higher and lower risks of death, respectively; the risk was significantly lower in patients who underwent laparoscopic surgery. Patients in both the advanced and nonadvanced CRC-LC groups had significantly higher hazard ratios (HRs) than did those in the LC group; however, the control and nonadvanced CRC groups had lower HRs than the LC group (Table 4).
This study examined the survival rates of healthy subjects, patients with LC alone, patients with CRC alone, and patients with both LC and CRC. Furthermore, the CRC patients' surgical and oncological outcomes were investigated based on the presence or absence of LC. Our findings suggest that LC determines the prognosis of patients with CRC regardless of the tumor's stage (i.e., advanced or nonadvanced). Additionally, the prognoses of patients without LC could depend on the stage of CRC. Given the paucity of research on the prognoses and postoperative outcomes of CRC patients with LC, this study had the advantage of investigating a national cohort.
The significantly high proportion of nonadvanced CRC in the CRC-LC group can be attributed to higher chances of discovering CRC in patients who have underlying LC. It is recommended that patients with LC seek routine screening for early detection of primary liver cancer [1415]; a periodic health screening program is available in South Korea for the general public [16]. A previous study found that the postoperative ICU admission rate in the CRC-LC group was 14.5%, which was significantly higher than the rate of 4.1% observed in the CRC group [4]. Our study showed the same trend, although the rates in both groups were higher (30.0% vs. 17.2%). This difference may be owing to the selection bias in single-institution studies and variations in ICU admission criteria.
Treating patients with both CRC and LC involves longer hospital stays and (consequently) higher medical costs [17]. Such patients also require continuous outpatient follow-up and necessary procedures more frequently than do patients with CRC patients who have no LC. As such, we observed that a higher SES (which was a prognostic factor in this study) was associated with a lower HR. The CCI has also previously been found to be a significant prognostic factor in patients with CRC [181920]. Likewise, the HR was significantly higher in patients with increasing CCIs.
Our multivariate analysis aimed at identifying factors that influence survival rates showed a significantly elevated HR when CRC occurred in patients with LC. There was a significant difference between the advanced CRC-LC group (HR, 1.858) and the nonadvanced-CRC-LC group (HR, 1.799); that both groups had HRs >1 indicated that CRC in patients with underlying LC was associated with a worsened prognosis regardless of CRC stage. Furthermore, the HR of the control group was 0.251 when using the LC group as a reference, which is consistent with data from previous studies that showed worsened prognoses in patients with CRC who had underlying LC than in those who do not. The nonadvanced CRC group had no survival advantages over the advanced CRC group, but did have a significantly lower HR (0.730) than that of the LC group. This indicates that LC has a greater influence on survival than CRC stage, and that the absence of LC in patients with CRC is associated with a significantly better prognosis.
This study also revealed significant differences in survival rates based on surgical methods and other prognostic factors. Laparoscopic surgery is currently performed for T4a and T4b cancers, and positive results are shown accordingly. However, the authors think that in the period of this study (2008–2013), open surgery was performed for T4a and T4b advanced CRC patients relatively and the prognostic factor was analyzed. Previous studies found that 7.3% and 12.7% of CRC patients with and without LC underwent laparoscopy, respectively [4]. Likewise, laparoscopy was significantly less frequent in our CRC-LC group (52.3%) than in our CRC group (59.9%). This difference is possibly attributable to a higher likelihood of poor prognosis in patients undergoing open surgery.
This study had some limitations. First, the nationwide data contain no information on recurrence or cancer stage. Some previous studies found that LC in patients with CRC influences the location of recurrence [2122]; however, we could not confirm this in our study. Patients with advanced and nonadvanced CRC in our study were examined separately based on the administration of cancer treatment. Moreover, 74%–78% of patients with CRC underwent postoperative cancer treatment, the administration of which is an indicator of cancer stage; as such, we modified the study design based on this indication [23]. Furthermore, the severity of LC was not investigated and we could not examine the differences in survival rates based on the commonly used Child-Pugh classification or the ‘model for end-stage liver disease’ score. In the future, we believe it is necessary to conduct research involving liver function.
In conclusion, patients with CRC who had underlying LC had a lower survival rate than did those without LC, although there were no differences in the incidences of postoperative complications. LC was associated with a significantly lower survival rate regardless of the CRC disease stage. Given the high postoperative mortality of patients with LC, caution is advised when planning and performing surgery; importantly, liver function (among other factors) should be taken into consideration. We also recommend careful postoperative management for patients with both CRC and underlying LC.
References
1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014; 383:1749–1761. PMID: 24480518.
2. Han EC, Ryoo SB, Park JW, Yi JW, Oh HK, Choe EK, et al. Oncologic and surgical outcomes in colorectal cancer patients with liver cirrhosis: a propensity-matched study. PLoS One. 2017; 12:e0178920. PMID: 28586376.
3. Friedman LS. Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc. 2010; 121:192–204. PMID: 20697561.
4. Nguyen GC, Correia AJ, Thuluvath PJ. The impact of cirrhosis and portal hypertension on mortality following colorectal surgery: a nationwide, population-based study. Dis Colon Rectum. 2009; 52:1367–1374. PMID: 19617746.
5. Lopez-Delgado JC, Ballus J, Esteve F, Betancur-Zambrano NL, Corral-Velez V, Manez R, et al. Outcomes of abdominal surgery in patients with liver cirrhosis. World J Gastroenterol. 2016; 22:2657–2667. PMID: 26973406.
6. Ziser A, Plevak DJ, Wiesner RH, Rakela J, Offord KP, Brown DL. Morbidity and mortality in cirrhotic patients undergoing anesthesia and surgery. Anesthesiology. 1999; 90:42–53. PMID: 9915311.
7. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.
8. Baek Y, Yi M. Factors influencing quality of life during chemotherapy for colorectal cancer patients in South Korea. J Korean Acad Nurs. 2015; 45:604–612. PMID: 26364535.
9. Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998; 28:921–925. PMID: 9755226.
10. Lee YH, Han K, Ko SH, Ko KS, Lee KU. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data analytic process of a nationwide population-based study using national health information database established by national health insurance service. Diabetes Metab J. 2016; 40:79–82. PMID: 26912157.
11. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005; 43:1130–1139. PMID: 16224307.
12. R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing;2013. Available online at https://www.R-project.org/.
13. An J, Won S, Lutz SM, Hecker J, Lange C. Effect of population stratification on SNP-by-environment interaction. Genet Epidemiol. 2019; 43:1046–1055. PMID: 31429121.
14. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014; 11:e1001624. PMID: 24691105.
15. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016; 150:835–853. PMID: 26795574.
16. Shim JI, Kim Y, Han MA, Lee HY, Choi KS, Jun JK, et al. Results of colorectal cancer screening of the national cancer screening program in Korea, 2008. Cancer Res Treat. 2010; 42:191–198. PMID: 21253320.
17. Csikesz NG, Nguyen LN, Tseng JF, Shah SA. Nationwide volume and mortality after elective surgery in cirrhotic patients. J Am Coll Surg. 2009; 208:96–103. PMID: 19228510.
18. Wu CC, Hsu TW, Chang CM, Yu CH, Lee CC. Age-adjusted Charlson comorbidity index scores as predictor of survival in colorectal cancer patients who underwent surgical resection and chemoradiation. Medicine (Baltimore). 2015; 94:e431. PMID: 25590852.
19. Chang CM, Yin WY, Wei CK, Wu CC, Su YC, Yu CH, et al. Adjusted age-adjusted Charlson comorbidity index score as a risk measure of perioperative mortality before cancer surgery. PLoS One. 2016; 11:e0148076. PMID: 26848761.
20. Laor A, Tal S, Guller V, Zbar AP, Mavor E. The Charlson Comorbidity Index (CCI) as a mortality predictor after surgery in elderly patients. Am Surg. 2016; 82:22–27. PMID: 26802847.
21. Utsunomiya T, Saitsu H, Saku M, Yoshida K, Matsumata T, Shimada M, et al. Rare occurrence of colorectal cancer metastasis in livers infected with hepatitis B or C virus. Am J Surg. 1999; 177:279–281. PMID: 10326842.
22. Song E, Chen J, Ou Q, Su F. Rare occurrence of metastatic colorectal cancers in livers with replicative hepatitis B infection. Am J Surg. 2001; 181:529–533. PMID: 11513779.
23. Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001; 93:850–857. PMID: 11390534.
Fig. 1
Kaplan-Meier plots of overall survival according to liver cirrhosis (LC) and colorectal cancer (CRC) status. N, no; Y, yes.

Fig. 2
Kaplan-Meier plots of overall survival according to the Charlson comorbidity index (CCI), location of colorectal cancer (CRC), socioeconomic status (SES), and method of CRC surgery. L, left; R, right.
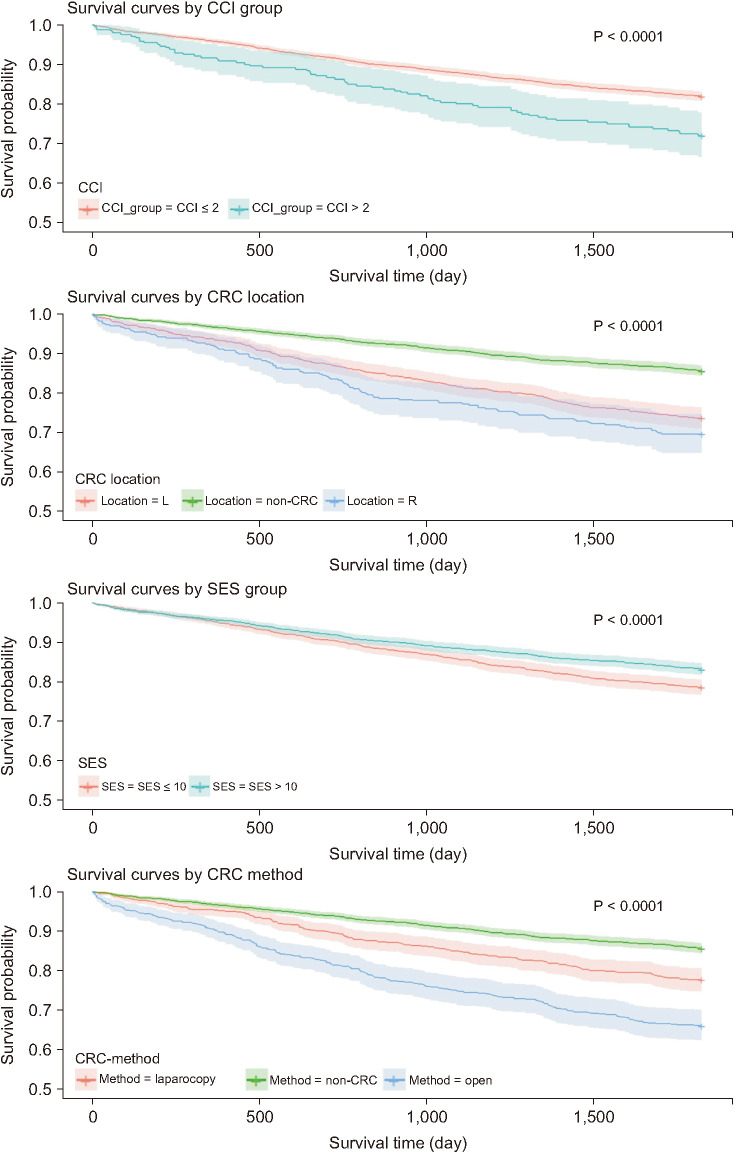
Table 1
Characteristics of colorectal cancer (CRC) patients with liver cirrhosis (LC) and without LC
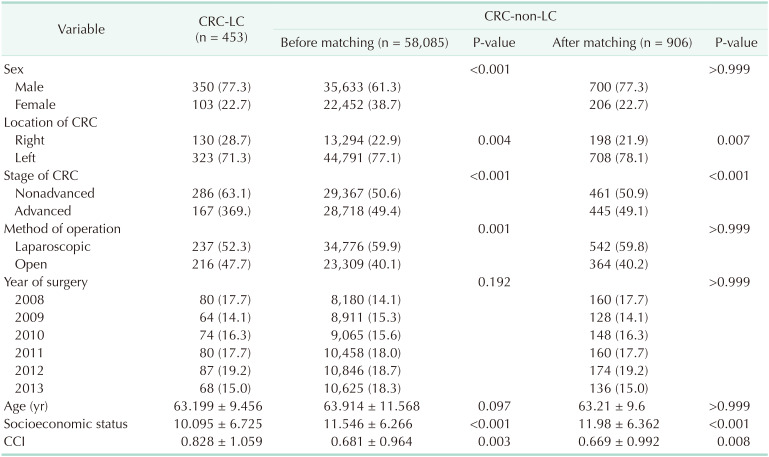
Table 3
Comparison of admission rate of ICU, incidence rate of incisional hernia, small bowel obstruction, and mortality rate after CRC surgery between non-LC and LC group after matching




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download