INTRODUCTION
The proportion of sphincter-preserving surgery for rectal cancer has increased dramatically in the past several decades with the development of the ‘double stapling technique’ since both circular and linear staplers allow for colorectal continuity in the deep pelvic cavity [
12]. The advancement of stapling devices has enabled safe standardized stapled anastomosis in clinical practice [
34]. The goal of good stapling technique is to achieve tissue apposition and hemostasis without tissue damage and ischemia [
5].
Despite the technical improvements, adverse events by circular stapler, including tissue damage, staple line defect, and malformed staples, have been reported in 19% in the clinical field [
5]. Particularly, tissue damage such as seromuscular rupture is not uncommon in the compression process when the colon wall had been weakened by colon cancer obstruction or preoperative chemoradiation therapy for rectal cancer [
26]. Recently, the U.S. Food and Drug Administration announced that over 41,000 individual medical device reports for surgical staplers accumulated during the last 7 years including 366 deaths, over 9,000 serious injuries, and over 32,000 malfunctions. As such, they have emphasized the importance of the proper use of surgical staplers to prevent surgical stapler-related casualties and serious malfunctions and improve patient safety [
7].
Tissue compression is an important step to ensure safe staple formation [
89]. The degree of proper compression is affected by variable tissue conditions such as tissue thickness, density, location, edema, and pathologic status [
8]. Surgeons usually consider these kinds of tissue conditions to obtain an optimal compression. However, such efforts sometimes do not completely eliminate the chance of accidental tissue damage during the compression process by circular stapler.
Improper usage of a circular stapler may result in tissue injury that could be considered an important risk factor for anastomotic leak [
38]. However, it is not easy for a surgeon to find tissue damage in the standardized stapling process. Especially, a crushing tissue injury on the compression line is often overlooked by surgeons because the stapled colon wall has been inverted to the luminal side which causes difficulty for the operator to notice the stapled tissue damage [
2]. That is why such tissue damage is not often identified during the rectal cancer surgery in the deep pelvic cavity. Thus, surgeons have not been interested in tissue compression injury due to circular staples, until recently. In addition, due to the lack of studies related to tissue compression injury due to circular staples, its damaging mechanism was not clearly identified. Therefore, the authors designed a preliminary
in-vitro study to quantitatively evaluate compression injury of circular stapling line on artificial collagen tissue.
This study was aimed to evaluate the risk factors and explore the mechanism of tissue compression injury from the use of a circular stapler for end-to-end anastomosis using in-vitro model.
Go to :

RESULTS
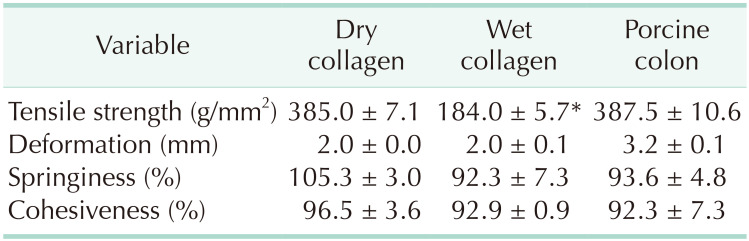
The physical properties of the collagen plates were compared with those of porcine colon tissue (
Table 1). The tensile strength and tissue texture such as deformation, springiness, and cohesiveness were comparable between the dry collagen plates and colon tissue. In the wet collagen plates, the tensile strength was significantly weaker than dry collagen or healthy colon tissue.
Table 1
Physical properties of dry and wet collagen plates compared with fresh porcine colon tissue

There were some structural differences between the 2 types of staplers. The height of pusher bar in the fixed-type stapler was significantly higher than those of the staple driver in the adjusting-type stapler (1.95 ± 0.01 mm vs. 0.73 ± 0.03 mm, P < 0.001). In the shape of anvil edge, a sharp lip protruded around the anvil of the fixed-type stapler and the length of sharp lip was 0.30 ± 0.01 mm. The adjustable-type stapler had an atraumatic round shape of the anvil edge.
The outcomes of
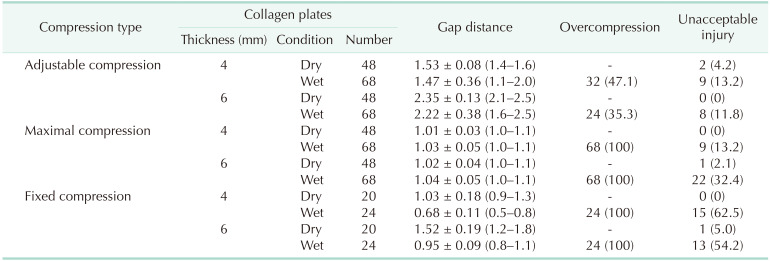
in-vitro experiments were different according to the collagen thickness and tissue conditions (
Table 2). In the adjustable compression, the compressibility ratio was similar between dry and wet collagen plates (61.7%
vs. 63.2%, respectively, P = 0.234). However, on the edematous collagen plates, the standard deviation ranges of gap distance were spread out more than 3 times those of dry plates. The rate of overcompression and unacceptable injury also had increased in the wet collagen. The overcompression cases had significantly lower gap distance and higher incidence of unacceptable injuries than those of optimal compression cases in the wet collagen plates (
Fig. 3). By maximal compression of the adjustable stapler, gap distance was intentionally made to 1 mm. Unacceptable injury was little on the dry collagen plates, but it increased to 32.4% on the wet, 6-mm thick collagen plates. In the fixed compression, gap distance was significantly narrower than that of adjustable compression (P < 0.001). The compressibility ratio increased significantly from dry collagen to wet condition (74.2%
vs. 83.1%, respectively, P < 0.001). Unacceptable injuries were observed in more than 50% of the wet collagens cases whereas only compressive impression or slight injuries were found on the dry collagen plates (
Table 2).
 | Fig. 3The gap distance and unacceptable injury of the adjustable type stapler according to compression propriety on the wet collagen plates. (A) Comparing with optimal compression, the overcompression cases had significantly lower gap distance. (B) The incidence of unacceptable injuries was higher in the overcompression cases than optimal compression cases. *P < 0.05.
|
Table 2
The gap distance, overcompression, and unacceptable injury rate according to the compression type

In the real-time compressive pressure measurements, the adjustable compression showed similar pressure patterns between the dry and wet collagen plates (
Fig. 4A). In the maximal or fixed compression, compressive pressures were markedly increased in the dry collagen plates than in the edematous collagen plates (
Fig. 4B, C). Peak compressive pressure levels showed significant increments in the fixed compression than those of adjustable compression in both dry and wet collagen plates (
Fig. 4D, E). As the gap distance was approximated in the adjustable compression, the upward curve patterns of compressive pressure were different depending on the collagen conditions. On the dry collagen plates, the compressive pressure curve showed a steep increase as the gap distance was closing. In contrast, the compressive pressure reached a gradual plateau even after reaching an optimal compression point on the wet collagen plates (
Fig. 4F).
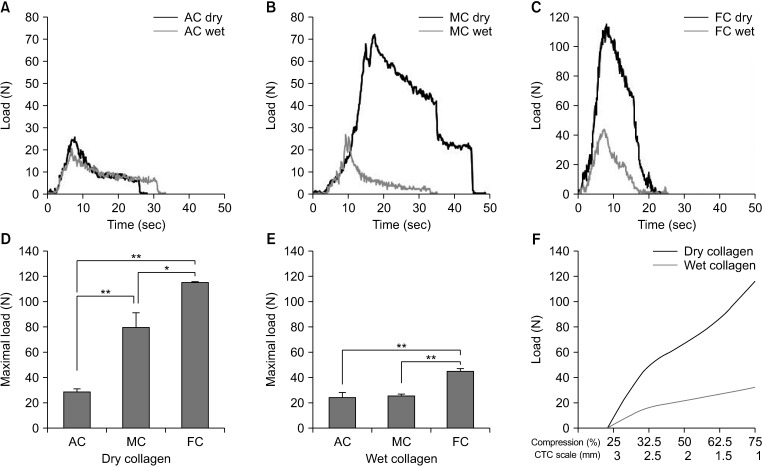 | Fig. 4Real-time compressive pressure measurements. Compressive pressure curves according to compression type of AC (A), MC (B), and FC (C) on the both dry and wet collagen plates. Peak compressive pressure levels showed significant increment in FC on the dry (D) and wet (E) collagen plates. (F) The upward curve patterns of compressive pressure were different on the collagen conditions. In contrary to steep pressure rising on the dry collagen plates, gradual plateau was showed even after optimal compression point on the wet collagen. AC, adjustable compression; MC, maximal compression; FC, fixed compression; CTC scale, controlled tissue compression scale. *P < 0.05, *P < 0.001.
|
On bivariate analysis of the risk factors relating to unacceptable injury, the fixed compression and wet conditioned plates were respectively correlated with overcompression which was considered as the prerequisite causing unacceptable injury (
Fig. 5).
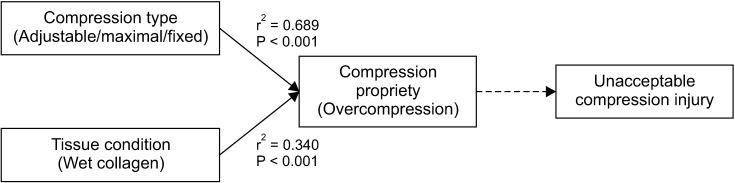 | Fig. 5The bivariate correlation analysis of the risk factors relating unacceptable injury.
|
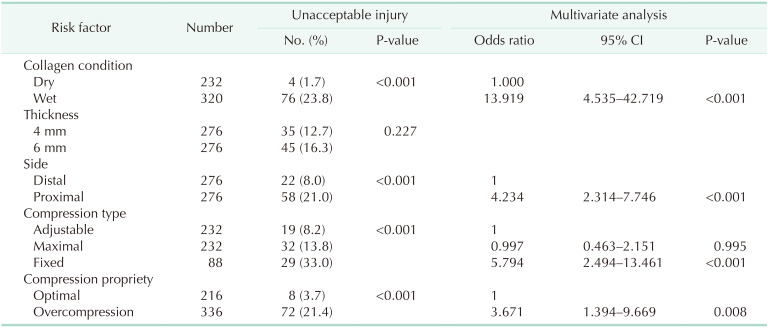
On univariate analysis, the rates of unacceptable injury were significantly higher in the wet collagen plates, proximal anvil side, fixed compression, and overcompression. On multivariate analysis, edematous collagen condition was the most important risk factor. The proximal anvil side, fixed compression, and overcompression were also analyzed as independent risk factors of unacceptable injury (
Table 3).
Table 3
Univariate and multivariate analysis for the risk factors of compression injury

Go to :

DISCUSSION
Despite the development of staple devices, it is not uncommon that tissue injury occurs on the staple line of edematous or friable bowel in the surgical field [
31112]. In this study, both adjustable and fixed compression were performed safely without damage on the dry collagen plates as normal healthy tissue. However, on the edematous wet plates, the rates of unintentional overcompression were up to 35%, and approximately 30% of them were associated with serious compression injury from adjustable compression. Fixed compression also caused overcompression in all cases, and tissue damage was observed in more than 50% of wet collagen plates. Even though there were different outcomes for tissue damage, edematous condition could be considered as the most significant risk factor for compression injury rather than the compression types of circular stapler device. Therefore, compression injury might be unavoidable on edematous tissue. However, understanding its mechanism of compression injury may help surgeons find the proper and safe usage of circular staplers.
In this study, the wet collagen plates and compression types were significantly related with overcompression which is considered an important prerequisite causing unacceptable compression injury. In order to evaluate the mechanism of the overcompression phenomenon, we measured the compression pressure patterns in proportion to the approximation of gap distance. In the adjustable compression device, the initial compression pressure increased until the gap distances reached almost 2 mm. The compression pressures vary between 60–70 N and these values were close to 6–8 g/mm
2, considering the target compressed area was 10 mm
2 in this model. Such compressive force is well associated with the stapler manufacturer's design concept to deliver 6–8 g/mm
2 of compressive force to the target area based on the previous researches seeking optimal compression [
81314].
In the dry collagen plates, a steep increase of compressive pressure was observed as gap distance reached the optimal point. This sudden change of compressive pressure serves as a useful indicator for the operator to recognize its optimal compression level. Healthy tissue has both a structural stability and viscoelasticity, which reflects its physical properties of high density with good elasticity. As the tissue is compressed slowly, the resistance force acting on the tissue surface against compression force is also slowly increased since the intercellular space inside the tissue is shrunken [
3]. This is a biomechanics perspective of how force is acting on tissue upon compression. Once the healthy tissue is gently, but completely, compressed, when the intercellular fluid is spilled out and tissue stiffness increase without cellular damage is established, the resistance force reaches the strongest point. The operator can perceive the abrupt resistance triggering turning of the rotating knob of the circular stapler device.
However, in the edematous collagen plates, the compressive pressure curve formed a gradual plateau even after further compression was applied beyond the optimal compression point. If there were presence of edema or inflammation in the colon, the tissue structure could be weakened and its density, elasticity, and restoration capability would have been deteriorated [
15]. As a result, its structural integrity could be even further weakened by the accumulation of muscular and submucosal tears caused by overcompression [
1216]. In the edematous tissue, its internal structure is not as stable as that of normal tissue and sufficient resistance force could not establish action on the tissue surface against compression force [
1718]. In this case, compressive pressure curve formed a plateau curve passing through the optimal point, even if compression is continued.
Thus, even an experienced operator could not often judge the optimal compression point on the edematous tissue using tactile feedback of compressive pressure, and it is easy to pass the optimal compression point inducing overcompression [
1718]. Therefore, the range of standard deviation for the gap distance was significantly wider in the wet collagen plates than that of dry collagen in this experiment. If excessive compression is applied beyond the optimal point, tissue damage could occur more frequently. That is why we have proposed this overcompression phenomenon as one of the key potential mechanisms causing compression injury in the edematous tissue. If surgeons fail to recognize the optimal compression point and apply overcompression, tissue damage could increase the risk of compression tissue injury and anastomotic complications [
3]. Therefore, a surgeon should be fully familiar with the proper usage of the circular stapler according to different tissue conditions since inappropriate usage could lead to a serious threat to patient safety [
317]. Also, we want to suggest that current adjustable compression based on the resistance feedback system may be suboptimal in preventing overcompression in edematous and friable tissue conditions.
The fixed compression device is designed to present a green bar indicator window of proper compression. Operators usually rotate the knob until the green bar is visualized, and that is the only indicator that proper compression has been achieved and the device is ready to fire. The gap distance of the fixed-compression type device was measured as shorter, at 0.5–0.8 mm, than the adjustable-type device on the dry collagen plates. There is a different method of measuring gap distance; using the CTC scale for the adjustable-type stapler and digital caliper for the fixed-type stapler. As such, almost 0.5 mm of anvil bucket and 0.3 mm of sharp lip on the anvil edge unit should be considered as an additional gap that might form similar gap distance ranges to the adjustable-type device. This could explain the comparable tissue injury rates on the dry collagen plates with the different compressibility of the 2 kinds of circular staplers.
Interestingly, the fixed-type compression caused more frequent tissue damage on the edematous tissue. In this study, researchers looked for additional causes of tissue damage from the structural differences between the fixed-type and adjustable-type devices. These different structural features of the circular staplers might have caused more frequent tissue damage on the edematous tissue. First, the fixed-type device had almost 10% excessive compression on the wet collagen plates than dry plates, but the adjustable-type device showed a similar level of compressibility between dry and wet collagen plates. Second, the fixed compression device has a sharp lip on the anvil edge while the adjustable device has an atraumatic round-shaped anvil. This edged structure appears to form dissipative frictional contact to limit tissue slippage in a radial direction during anvil approximation, which could prevent tissue elongation maintaining original tissue thickness and might have function in limiting maximal tissue compression [
18]. Unfortunately, this anvil's sharp lip could cause shearing on the edematous tissue. Third, there were different sized staple stationary units in the cartridges of the fixed and adjustable-type devices. The cartridges of fixed and adjustable-type instruments are equipped with a staple driver or pusher bar as homologous devices for staple stationary units. This staple driver holds the staple inside the cartridge and pushes staples towards the anvil bucket to form a B shape when the firing trigger is closed. However, in the fixed-type device, the pusher bar was more elevated than the staple driver of the adjustable-type devices when the trigger was fully closed. These different structural features of circular staplers might have caused more frequent tissue damage on the edematous tissue.
On the dry collagen conditions which represent normal tissue, the performance of both circular stapler devices was safe and acceptable. However, with edematous or inflammatory conditions, the different device structures could act as a dangerous trap with improper usage in the compression process. Therefore, the authors would like to suggest that structural aspects relating to the potential risk of tissue damage should be improved upon in the development of new circular staples.
This study had several limitations. First, the single-layered collagen plate is a simple structure compared to multi-layered colon tissue. It is possible that compression injury in the collagen plate may be exaggerated when it is compared to edematous colon tissue [
19]. On the physical property test, tensile strength was similar between dry collagen and healthy colon tissue. Thus, unacceptable compression injuries were rarely seen in the dry collagen plate regardless of compression type. However, the edematous collagen plate had significantly lower tensile strength than those of the dry collagen and healthy colon tissue. Therefore, the wet collagen plate was considered to be more susceptible to crushing injury. However, the collagen model differs from actual colon tissue in several aspects. The collagen model has a single layer structure of mixed components of collagen and glycerin, whereas the large intestine tissue has a multilayer structure with different thicknesses and physical properties. Therefore, the degree of tissue compression and its damage on the collagen plates must also be different from the actual colon tissue. Therefore, the results of this
in-vitro study need to be verified in real colon tissues. In addition, since animal colon and human colon tissues also have different thicknesses and strengths, subsequent studies need to be continued using human colon tissues obtained during surgery. In spite of these structural differences, collagen plates have some advantages on simulating edematous tissue conditions simply by soaking it in tap water for 2 minutes. Also, the transparent plate makes it possible to provide visual feedback of tissue injury and to assess the degree of crushing damage. This collagen plate model is also inexpensive, easy to manufacture, and free from ethical constraints which make it possible to carry out repetitive exercises for educational information regarding the safe use of circular staplers. Therefore, the collagen plates could be considered an effective training tool in learning how to determine optimal compression of circular staplers for novices using a circular stapler in real practice. Second, this study did not assess the tissue perfusion and bursting strength of stapled tissue. Optimal tissue compression should avoid tissue damage and maintain adequate tensile strength with adequate blood flow [
20]. Third, we performed only full compression of the fixed-type stapler until the green bar became fully visualized in this study. Therefore, experiments using partial visualization as adjustable compression were not conducted for EEA devices in this study. The instability of fixed-type staplers may have originated from specifically determinant ways of using complete visualization of the green bar for proper compression. The instructions for users (IFU) of EEA circular staplers described that the green bar must be visible in the indicator window before releasing the safety lever prior to firing. The in-service educational information of EEA device recommends that full visualization of the green bar indicates that the stapler is ready to be fired; which is generally used as the proper compression indicator during surgery. However, even if only a part of the green bar is visible, the EEA device is designed to enable firing. The authors had some experiences of safe stapling of EEA when the green bar was partially visible on thick colon tissues in the surgical field. This partial visualization related patent was registered and that novel indicator window was designed to enable adjustable compression, but this patent technology has not been applied to the commercial EEA device until now [
21]. Therefore, even if the fixed-type stapler is used, firing may be attempted when colon tissue can be compressed comfortably at appropriate compression levels. However, since the indicator window for viewing the compression scale is narrow and there is no detailed mention of using partial or full visualization of the green bar on the indicator window for optimal compression in the IFU of the EEA device, inexperienced users still could have uncertainty in achieving optimal compression using circular staplers. Therefore, the researchers eagerly hope for the advancement and development of circular stapler devices in which the operator can easily and clearly recognize optimal compression levels using a distinctive indicator.
This was a preliminary in-vitro study on the effect of compression injury of the circular stapling line on artificial collagen tissue. Therefore, the results based on quantitative evaluation of compression injury suggest additional ex-vivo evaluations using real colon tissue in the future. Clinical studies also should be conducted to identify the clinical implications of crushing injuries caused by overcompression of circular staplers. Also, further clinical studies should be designed to help develop effective training programs for the standardization and optimization for proper application of staple devices.
In edematous tissue conditions, unintentional overcompression could be increased and result in unacceptable tissue injury on the compression line of circular staplers. Well-designed training programs for new device users are required to teach proper usage of circular staplers to minimize compression injury at the stapling line to ensure safe anastomosis.
Go to :








 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download