1. Thongprayoon C, Kaewput W, Thamcharoen N, Bathini T, Watthanasuntorn K, Lertjitbanjong P, et al. Incidence and impact of acute kidney injury after liver transplantation: a meta-analysis. J Clin Med. 2019; 8:372.
2. Bahirwani R, Reddy KR. Outcomes after liver transplantation: chronic kidney disease. Liver Transpl. 2009; 15 Suppl 2:S70–S74. PMID:
19876956.
3. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003; 349:931–940. PMID:
12954741.
4. Kim HY, Lee JE, Ko JS, Gwak MS, Lee SK, Kim GS. Intraoperative management of liver transplant recipients having severe renal dysfunction: results of 42 cases. Ann Surg Treat Res. 2018; 95:45–53. PMID:
29963539.
5. Chen J, Singhapricha T, Hu KQ, Hong JC, Steadman RH, Busuttil RW, et al. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: a matched study. Transplantation. 2011; 91:348–353. PMID:
21127462.
6. Utsumi M, Umeda Y, Sadamori H, Nagasaka T, Takaki A, Matsuda H, et al. Risk factors for acute renal injury in living donor liver transplantation: evaluation of the RIFLE criteria. Transpl Int. 2013; 26:842–852. PMID:
23855657.
7. Hilmi IA, Damian D, Al-Khafaji A, Planinsic R, Boucek C, Sakai T, et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth. 2015; 114:919–926. PMID:
25673576.
8. Leithead JA, Ferguson JW, Bates CM, Davidson JS, Simpson KJ, Hayes PC. Chronic kidney disease after liver transplantation for acute liver failure is not associated with perioperative renal dysfunction. Am J Transplant. 2011; 11:1905–1915. PMID:
21827620.
9. Rana A, Petrowsky H, Hong JC, Agopian VG, Kaldas FM, Farmer D, et al. Blood transfusion requirement during liver transplantation is an important risk factor for mortality. J Am Coll Surg. 2013; 216:902–907. PMID:
23478547.
10. Richer M, Robert S, Lebel M. Renal hemodynamics during norepinephrine and low-dose dopamine infusions in man. Crit Care Med. 1996; 24:1150–1156. PMID:
8674327.
11. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145:247–254. PMID:
16908915.
12. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11:R31. PMID:
17331245.
13. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39(2 Suppl 1):S1–S266. PMID:
11904577.
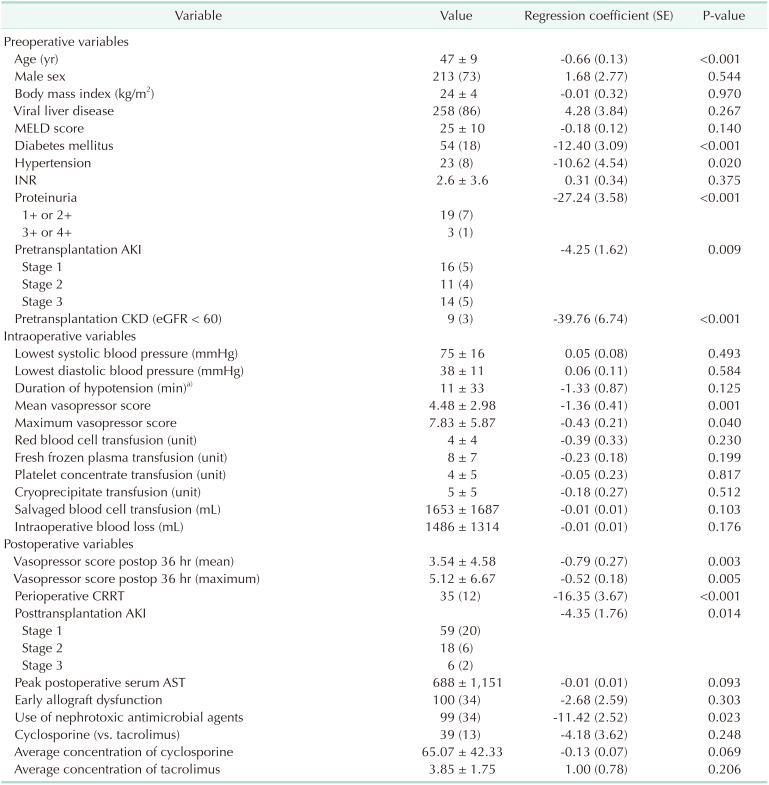
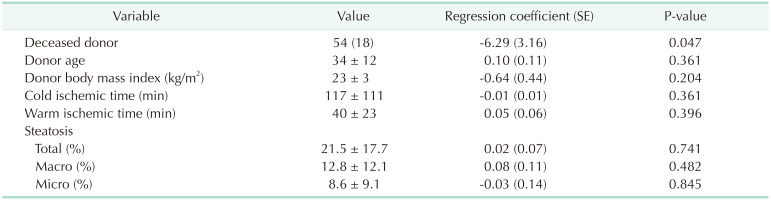
14. Bang SR, Ahn HJ, Kim GS, Yang M, Gwak MS, Ko JS, et al. Predictors of high intraoperative blood loss derived by simple and objective method in adult living donor liver transplantation. Transplant Proc. 2010; 42:4148–4150. PMID:
21168648.
15. Zuppa AF, Nadkarni V, Davis L, Adamson PC, Helfaer MA, Elliott MR, et al. The effect of a thyroid hormone infusion on vasopressor support in critically ill children with cessation of neurologic function. Crit Care Med. 2004; 32:2318–2322. PMID:
15640648.
16. Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010; 16:943–949. PMID:
20677285.
17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007; 370:1453–1457. PMID:
18064739.
18. Saliba F, Fischer L, de Simone P, Bernhardt P, Bader G, Fung J. Association between renal dysfunction and major adverse cardiac events after liver transplantation: evidence from an Internat ional Randomized Trial of Everolimus-Based Immunosuppression. Ann Transplant. 2018; 23:751–757. PMID:
30361470.
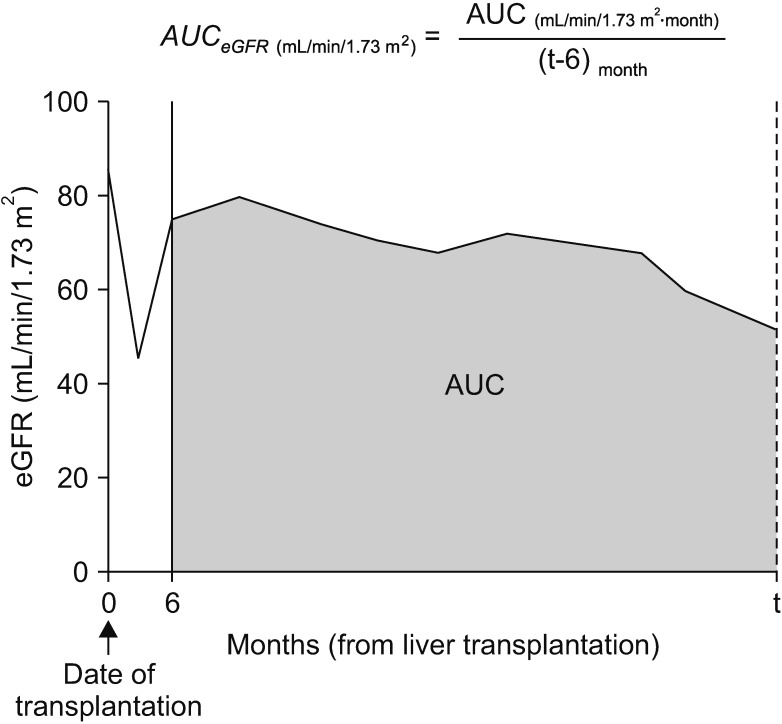
19. Yilmaz S, Yilmaz A, Häyry P. Chronic renal allograft rejection can be predicted by area under the serum creatinine versus time curve (AUCCr). Kidney Int. 1995; 48:251–258. PMID:
7564086.
20. Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014; 311:2518–2531. PMID:
24892770.
21. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013; 382:260–272. PMID:
23727169.
22. Rifkin DE, Coca SG, Kalantar-Zadeh K. Does AKI truly lead to CKD? J Am Soc Nephrol. 2012; 23:979–984. PMID:
22460531.
23. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001; 281:F887–F899. PMID:
11592947.
24. Basile DP, Leonard EC, Tonade D, Friedrich JL, Goenka S. Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion. Am J Physiol Renal Physiol. 2012; 302:F625–F635. PMID:
22114210.
25. Northup PG, Argo CK, Bakhru MR, Schmitt TM, Berg CL, Rosner MH. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl. 2010; 16:440–446. PMID:
20205164.
26. Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012; 81:477–485. PMID:
22157656.
27. Kellum JA. Persistent acute kidney injury. Crit Care Med. 2015; 43:1785–1786. PMID:
26181122.
28. Xia VW, Du B, Braunfeld M, Neelakanta G, Hu KQ, Nourmand H, et al. Preoperative characteristics and intraoperative transfusion and vasopressor requirements in patients with low vs. high MELD scores. Liver Transpl. 2006; 12:614–620. PMID:
16555319.
29. Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med. 2008; 36(4 Suppl):S179–S186. PMID:
18382191.
30. Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007; 107:213–220. PMID:
17667564.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download