Abstract
Purpose
Methods
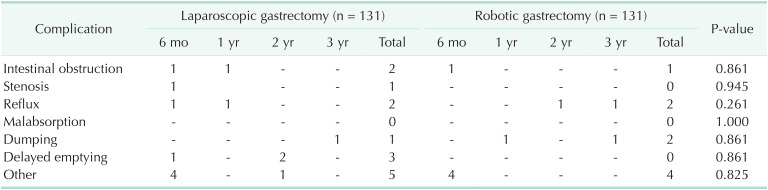
Results
Notes
Fund/Grant Support: This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1020410).
Author Contribution:
Conceptualization: HJL, WJH, SUH, MCK, SP.
Formal Analysis: JHC, HKY, HJL, SHK.
Investigation: YWK, KWR, JMP, JYA, KYS, SJO, BJS, DHY, TKH, HIK.
Methodology: HJL, WJH, HIK.
Project Administration: HJL.
Writing — Original Draft: JHC, HJL.
Writing — Review & Editing: SUH, HKY, YWK, KWR, JMP, JYA, MCK, SP, KYS, SJO, SHK, BJS, DHY, TKH, HIK, WJH.
References
Fig. 1
Quality of life (QOL) survey. (A) The schedule of the QOL survey. Five surveys were completed at the time of preoperative admission, postoperative 1st outpatient department (OPD) visit after discharge, and 3 months, 1 year, and 3 years. The answers were collected from 337, 302, 157, 220, and 165 patients at the respective time points. (B) The response of the survey. Robot multicenter study collected surveys from 434 patients from 11 centers, and 113 patients did not answer both preoperative and postoperative surveys. Accordingly, 321 cases were used as the initial data set for the following analyses.

Fig. 2
Quality of life (QOL) of laparoscopic surgery vs. robotic surgery. The QOL questionnaire (QLQ)-C30 and QLQ-STO22 developed by the European Organization for Research and Treatment of Cancer were used. A total of 24 scales are presented in the graph. In the respective scale, the maximum score is 100 and the minimum score is 0. A high score of general health status and 5 functioning scales indicates a good QOL. A high score of 18 symptom scales indicates a poor QOL. LG, laparoscopic gastrectomy; RG, robotic gastrectomy; Preop, preoperative; OPD, outpatient department.
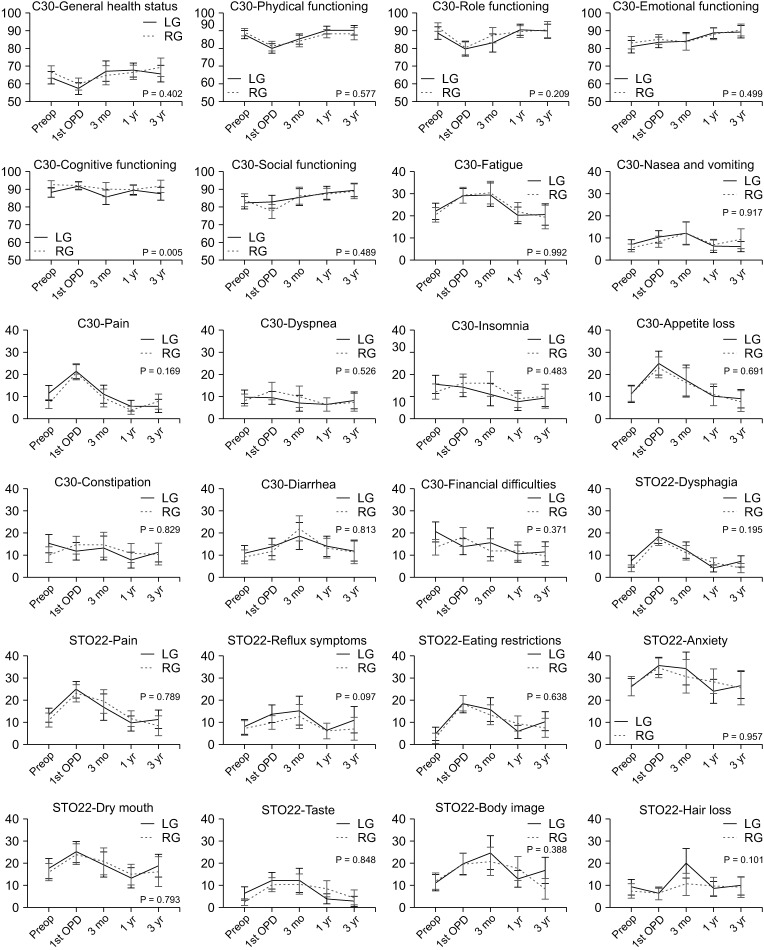
Fig. 3
Change of pain score and the use of pain killers. (A) Resting pain score from operation day (OP-day) to discharge (P = 0.614). (B) Movement pain score (P = 0.421). (C) The use of painkiller (frequency, P = 0.824). (D) The use of patient-controlled analgesia (PCA) (volume, P = 0.524). VAS, visual analog scale; LG, laparoscopic gastrectomy; RG, robotic gastrectomy; POD, postoperative day.
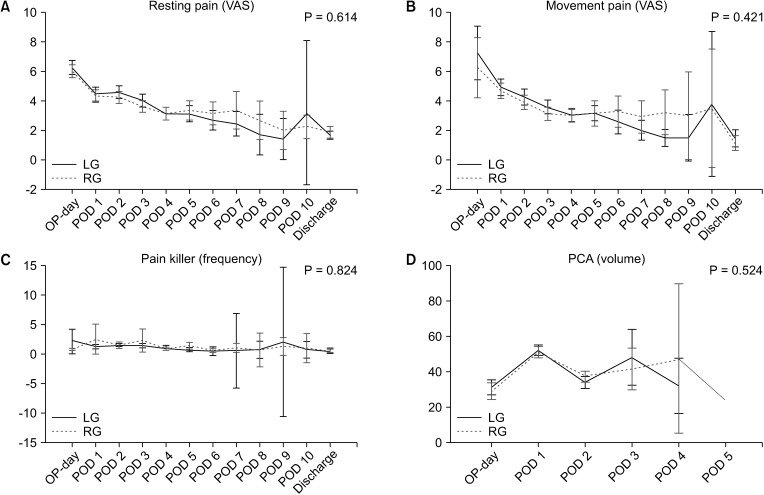
Table 1
Demographics of participants
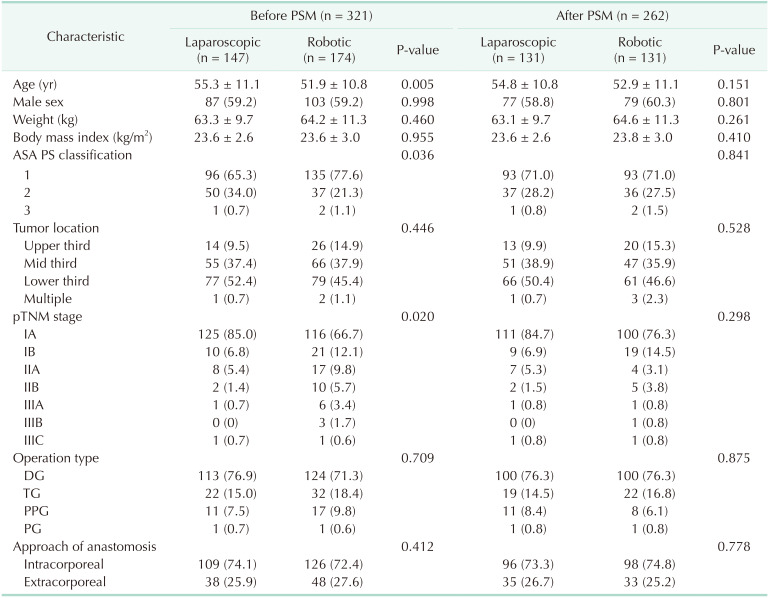
Values are presented as mean ± standard deviation or number (%).
We used propensity score matching (PSM) to correct selection bias because the patient's demographics were heterogeneous.
ASA, American Society of Anesthesiologists; PS, physical status; pTNM, pathologic TNM; DG, distal gastrectomy; TG, total gastrectomy; PPG, pylorus-preserving gastrectomy; PG, proximal gastrectomy.
Table 3
Analysis of the factors that cause the difference in quality of life (QOL)
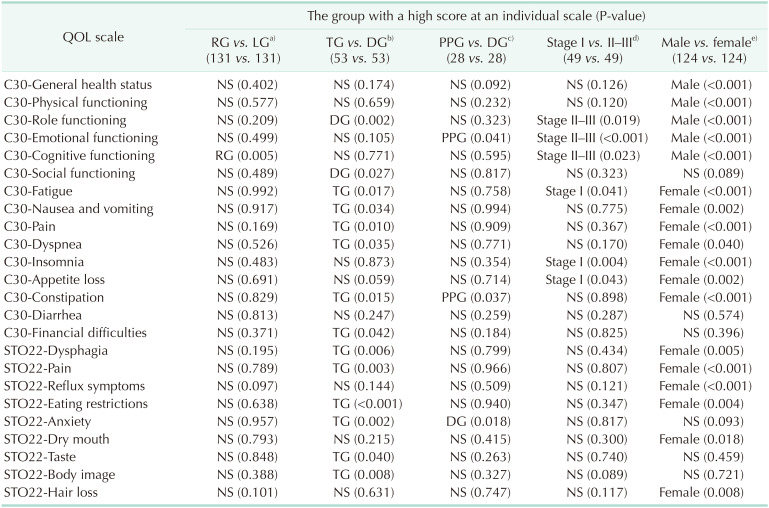
The QOL questionnaire (QLQ)-C30 and QLQ-STO22 developed by the European Organization for Research and Treatment of Cancer were used for the QOL survey.
RG, robotic gastrectomy; LG, laparoscopic gastrectomy; TG, total gastrectomy; DG, distal gastrectomy; PPG, pylorus-preserving gastrectomy; NS, not significant (P ≥ 0.05).
a)A comparison between RG and LG was adjusted by propensity score matching with age, American Society of Anesthesiologists physical status classification, and pathologic TNM (174 vs. 147 → 131 vs. 131). b)A comparison between TG and DG was matched with 2 confounders, weight and approach of the anastomosis (54 vs. 237 → 53 vs. 53). c)A comparison between PPG and DG was matched with weight and approach of the anastomosis (28 vs. 237 → 28 vs. 28). d)A comparison between stage I and stage II–III was matched without a confounder (272 vs. 49 → 49 vs. 49). e)A comparison between male and female patients was matched with body mass index (190 vs. 131 → 124 vs. 124).
Table 4
Chronological change of quality of life (QOL): compare of 4 study groups
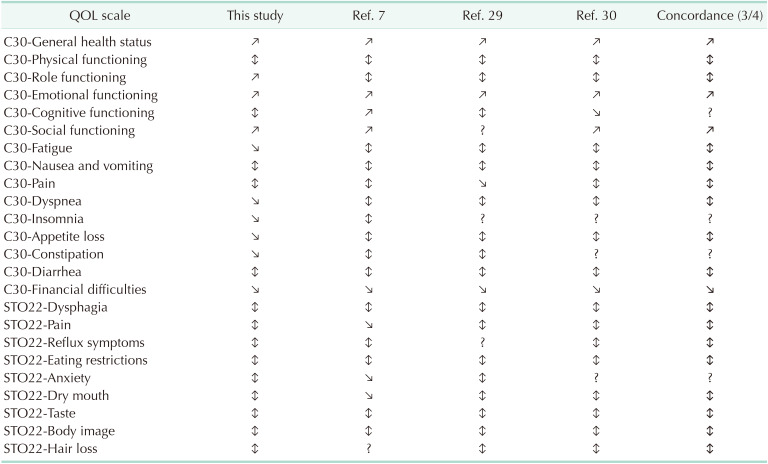
The QOL questionnaire (QLQ)-C30 and QLQ-STO22 developed by the European Organization for Research and Treatment of Cancer were used for the QOL survey.
Ref, reference; ↕, recovery (function scale decreased and partially recovered, symptom scale increased and partially recovered); ↗, increase (function scale finally over baseline); ↘, decrease (symptom scale finally under baseline).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download