Abstract
Purpose
Visfatin is a key cytokine released from the pe ripheral blood mononuclear cells (PBMCs) as well as adipose tissue, and it is involved in immune response as well as inflammation. In this study, we investigated whether the serum visfatin level could be a prognostic factor for predicting the severity of inflammation in patients with acute cholecystitis.
Methods
We examined the blood samples and gallbladder specimens from patients who underwent laparoscopic cholecystectomy for either acute (n = 18) or chronic cholecystitis (n = 18). We determined the visfatin levels of these samples using various procedures such as real-time polymerase chain reaction, enzyme-linked immunosorbent assay, western blotting, and immunohistochemistry.
Results
The patients with acute cholecystitis exhibited higher mRNA expression of visfatin in PBMCs, higher serum levels of visfatin, and increased protein expression of visfatin in the gallbladder specimens than in patients with chronic cholecystitis. In the in vitro model of acute cholecystitis, the mRNA expression of visfatin showed the fastest increase among the other pro-inflammatory mediators studied, including interleukin (IL)-10, tumor necrosis factor-α, IL-6, intracellular adhesion molecule-1, and ascular cell adhesion molecule-1. Inhibition of visfatin using siRNA abrogated the inhibitory effects of lipopolysaccharide (LPS) on the expression of ABCG1 in GBECs, suggesting that visfatin is significantly involved in the LPS-driven suppression of ABCG1.
Conclusion
Taken together, we concluded that visfatin is a pro-inflammatory mediators that is upregulated during acute cholecystitis and is expected to be increased within a short time after inflammation. Therefore, measuring the serum level of visfatin would be helpful in predicting the inflammatory severity in the patients with acute cholecystitis.
Acute cholecystitis is an acute inflammation of the gallbladder caused by the occlusion of the cystic duct, mainly in gallstone patients. Acute cholecystitis is a common clinical situation, accounting for up to 5% of emergency room visits and 9% of hospital admissions [1]. Acute cholecystitis is the most common complication of gallstones and has been reported to occur in 6%–11% of patients with symptomatic gallstones after 7 to 11 years [2]. It is also the second most common cause of complicated intraperitoneal infections [3]. Thus, acute cholecystitis is a disease that, when not treated, can spread to systemic inflammation and lead to sepsis, organ failure, and even death. In patients diagnosed with the same acute cholecystitis, the severity varies considerably, from simple inflammation of the gallbladder wall, to complications confined to the nearby area, and to accompanying multiple organ failure. In some cases, acute cholecystitis can easily progress to complicated cholecystitis that undergoes secondary changes, such as hemorrhage, gangrenous formation, and perforation within a short time. However, at present, it is difficult to predict whether the patients with acute cholecystitis have complicated or uncomplicated course. Therefore, it is crucial to devise methods to predict the progression of acute cholecystitis in the patients.
Xie et al. [4] reported that elevated plasma visfatin levels correlate with the conversion rate of laparoscopic cholecystectomy to open surgery in acute cholecystitis. Visfatin, which has been known as a pre-B cell colony-enhancing factor, was first isolated from visceral fat tissue by Fukuhara et al. [5]. Further studies revealed that visfatin is released from not only visceral fat but also from subcutaneous fat in humans [6]. Collectively, visfatin is released by peripheral blood cells, such as B cells, T cells, macrophages, and neutrophils, as well as fat tissues throughout the body [78]. Although its role has not been completely determined, several studies support that visfatin has glucose-lowering effects similar to insulin and it is essentially involved in immune response and inflammation [91011]. Visfatin is synthesized by the interaction of several cytokines, such as interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-6, and is also upregulated by lipopolysaccharide (LPS) and dexamethasone [1213]. Expression of visfatin is elevated in numerous inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, psoriasis, acute lung injury, and sepsis in which visfatin sustains the inflammation by inhibiting neutrophil apoptosis [1012141516]. In response to an inflammatory reaction, visfatin is elevated in the lymphocytes and promotes proliferation of lymphocytes [17]. In this study, we investigated the significance of visfatin in the progression of acute cholecystitis by evaluating the changes in the visfatin levels compared to those of various inflammatory mediators during acute cholecystitis.
Acute and chronic cholecystites were defined by a histological finding of an acute and chronic inflammatory infiltrates on examination of the gallbladder wall, respectively. When there was a concurrent acute and chronic inflammatory infiltrate, this was also included in the acute cholecystitis. We collected gallbladder specimens and blood samples from patients who underwent laparoscopic cholecystectomy due to acute cholecystitis (n = 18) or chronic cholecystitis (n = 18) at Daejeon St. Mary's hospital, the Catholic University of Korea. This research was approved by Institutional Review Board (IRB number: 2014-0319-0002) of the Catholic University of Korea. All patients who participated in the study were informed of the study and submitted informed consent. The blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tube and separated using a centrifuge (Hanil; Combi R515, Gimpo, Korea). Peripheral blood mononuclear cells (PBMCs) are separated from the whole blood by a density gradient centrifugation method using Ficoll Hisopague 1077 (Sigma-Aldrich, St. Louis, MO, USA).
Human gallbladders were obtained from patients undergoing cholecystectomy at the Catholic University of Korea. Isolation and primary culture of human gallbladder epithelial cells (GBECs) were followed by the instruction previously described [18]. Briefly, GBECs were isolated from the gallbladders by trypsin digestion. Resected gallbladder tissues were immediately transferred into ice-cold Ca2+-free Hanks solution with 0.5 mM EGTA, cut into 0.5 cm2 in size under sterile condition, and treated for 45 minutes at room temperature with 0.025% trypsin in phosphate-buffered saline containing 0.02% EDTA-2Na. Tissues were agitated by pipetting. The released cells were collected after centrifugation at 1,200 × g centrifuge for 5 minutes. The released cells were seeded on 35-mm culture dishes. The cells were maintained in DMEM/F12 (Dulbecco's Modified Eagle Medium/Ham's F-12; Thermo, Carlsbad, CA, USA). The medium was supplemented with 10% fetal bovine serum (GibcoBRL, Calsbad, CA, USA) and 1% antibiotics (Thermo) at 37℃. Finally, we generated an in vitro model of acute cholecystitis by treating human GBECs with 25-ng/mL LPS for 4 hours.
Total cell RNA was extracted using FavorPrep TRI-RNA reagent (Favorgen, Ping-Tung, Taiwan) according to the manufacturer's instructions. Reverse transcription was performed with 1 µg RNA using ReverTra Ace qPCR RT Master mix (TOYOBO, Osaka, Japan) according to the manufacturer's instructions. The primers used for SYBR Green real-time quantitative (q) polymerase chain reaction (PCR) were as follows: TNF-α, sense, R: 5′-ATGGGCTACAGGCTTGTCACT-3′ and antisense, F: 5′-CCCAGGGACCTCTCTCTAATC-3′; IL-10, sense, R: 5′-TCTTGGAGCTTATTAAAGGCATTC-3′ and antisense, F: 5′-CATCGATTTCTTCCCTGTGAA-3′; intrac ellular adhesion molecule-1 (ICAM-1), sense, R: 5′-TTATGACTGCGGCTGCTACC-3′ and antisense, F: 5′-AGCACTCAAGGGGAGGTCAC-3′; and GAPDH, sense, R: 5′-TGGCTCTCGCTGTTGAAGTC-3′ and antisense, F: 5′-ATCATCCCTGCCTCTACTGG-3′. The reaction was performed using an Applied Biosystems 7500 96-Well Real-Time PCR system (Life Technologies, Carlsbad, CA, USA). After normalization to the GAPDH gene, the expression levels for each target gene were calculated using the comparative threshold cycle method.
Transcription was specifically suppressed by the introduction of the 21-nucleotide duplex siRNA, which targeted visfatin mRNA coding sequence. Briefly, GEBP cells were plated in 6-well plates (2 × 105 cells/well) and transiently transfected with 100 nM visfatin siRNA (Thermo Fisher Scientific, Waltham, MA, USA) mixed with the LipofectamineTM RNAiMAX transfection reagent (Thermo Fisher Scientific) per each well according to the manufacturer's instructions.
After centrifugation of blood samples at 2,000 × g for 10 minutes, serum was collected. The concentrations of visfatin (Biovision, Milpitas, CA, USA), IL-6 (Biolegend, San Diego, CA, USA), resistin (Biocompare, San Francisco, CA, USA), and ICAM-1 (Biocompare) were measured by sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions.
GBECs and gallbladder specimens were lysed using the EzRIPA Lysis kit (ATTO Corp., Tokyo, Japan), and quantified by Bradford reagent (Bio-Rad, Hercules, CA, USA). Proteins were visualized by western analysis using the following primary antibodies (1:1000 dilution) at 4℃ overnight and then with Horseradish Peroxidase (HRP)-conjugated secondary antibodies (1:2000 dilution) for 1 hour at 25℃. The primary antibodies used in this study include visfatin, F4/80, and β-actin. (from Cell Signaling Technology, Beverly, MA, USA). Specific immune complexes were detected using the Western Blotting Plus Chemiluminescence Reagent (Millipore, Bedford, MA, USA).
For immunohistochemical (IHC) analysis, formalin-fixed, paraffin-embedded tissue sections were deparaffinized, rehydrated in an ethanol series and subjected to epitope retrieval using standard procedures. Antibodies used herein include the antibodies against visfatin (Abcam, Cambridge, England), CD68 (Abcam), ICAM-1 (Abcam), and vascular cell adhesion molecule-1 (VCAM-1; Santa Cruz Biotechnology, Santa Cruz, CA). The samples were then examined under a laser-scanning microscope (Eclipse TE300; Nikon, Tokyo, Japan).
For IHC analysis, formalin-fixed, paraffin-embedded tissue sections were deparaffinized, rehydrated in an ethanol series. Epitope retrieval was done in ph6.0 citrate buffer in a microwave for 10 minutes. Primary antibodies used herein include the antibodies against visfatin (Abcam), CD68 (Abcam), ICAM-1 (Abcam), and VCAM-1 (Santa Cruz Biotechnology). Negative control was made by replacing primary antibody by tris buffer, positive control was made gallbladder cancer tissue, kidney tissue, and human tonsil tissue. Next, tissue slide was incubated biotinylated secondary antibody (Vectastatin Elite ABC HRP kit, Vector Laboratories, Burlingame, CA, USA) at room temperature. Immunoreactivity was visualized using Vector imm PACT NovaRED substrate kit (Vector Laboratories). The samples were then examined under a laser-scanning microscope (Eclipse TE300, Nikon). The dyeing area was measured at random. The slides were evaluated 3 times.
All data were analyzed with SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA), and are presented as mean ± standard deviation. Statistical comparison among groups was determined using Kruskal-Wallis test followed by Dunnett test as the post hoc analysis. Probability values of P < 0.05 were regarded as statistically significant.
In this study, we attempted to find the most sensitive inf lammatory marker that is elevated during acute cholecystitis. First, we examined the differences between the mRNA expression levels of pro-inflammatory mediators, including visfatin, ICAM-1, VCAM-1, and resistin, in the PBMCs of the patients with chronic and acute cholecystitis (Fig. 1A). We collected blood from patients planned to undergo cholecystectomy the day before surgery to measure these variables. We found that the mRNA levels of these pro-inflammatory markers were significantly higher in the PBMCs of patients with acute cholecystitis than in the chronic cholecystitis patients (P < 0.05). Out of all the markers, resistin and visfatin showed the higher increase compared to the expression levels of ICAM-1 and VCAM-1.
We next compared the serum levels of pro-inflammatory mediators, such as visfatin, IL-6, resistin, and ICAM-1, in the sera of each patient group (Fig. 1B). It was found that the serum levels of these markers were significantly higher in acute cholecystitis patients with in chronic cholecystitis patients (P < 0.05). In addition, serum levels of visfatin were significantly higher than those of other pro-inflammatory mediators (P < 0.05).
Next, we compared the expression of pro-inflammatory proteins (visfatin and F4/80) in the gallbladder specimens between patients with acute cholecystitis and chronic cholecystitis using western blot analysis (Fig. 2). We obtained 2 areas of grossly inflamed and noninflammated gallbladder tissues, respectively, from patients with acute cholecystitis and chronic cholecystitis. In the gallbladder tissues with chronic cholecystitis, the expression levels of visfatin and F4/80 did not significantly differ between grossly inflamed and noninflamed tissues. However, in the gallbladder tissues with acute cholecystitis, the expression levels of visfatin and F4/80 were significantly higher in grossly inflamed tissues than in grossly noninflamed tissues (P < 0.05).
Subsequently, we compared IHC staining of the gallbladder specimens in each group (Fig. 3). The antibodies used for IHC stains included antibodies against visfatin, CD68, ICAM-1, and VCAM-1. The percentages of immunoreactive areas were measured using the ImageJ software (National Institute Health, Bethesda, MD, USA) and were expressed as values relative to those in normal gallbladder. IHC staining showed that immunoreactive areas for these pro-inflammatory mediators were significantly higher in the gallbladder specimens, especially grossly inflamed tissues, of the patients with acute cholecystitis than those of the patients with chronic cholecystitis (P < 0.05).
To generate the in vitro model of acute cholecystitis, we investigated the mRNA expression of pro-inflammatory mediators, including visfatin, IL-10, TNF-α, IL-6, ICAM-1, and VCAM-1 according to the varying concentration of LPS (Fig. 4A). As LPS had higher concentrations, the mRNA expression of these pro-inflammatory mediators tended to increase, and thereafter the mRNA expression was shown to be decreased. We also found that most pro-inflammatory mediators significantly increased when the LPS of concentration above 25-ng/mL concentration was added. Based on these findings, we use the 25-ng/mL LPS to generate the in vitro model of acute cholecystitis.
After generating the in vitro model for acute cholecystitis, we compared the time-dependent differences in the levels of these pro-inflammatory mediators using real-time PCR (Fig. 4B). The results of the real-time PCR demonstrated that the mRNA expression of visfatin was increased in the shortest time span compared to those of the other pro-inflammatory mediators. Contrasted by other pro-inflammatory mediators, the mRNA expression of visfatin was increased within 1 hour after LPS-driven inflammation.
To determine the role of visfatin, we compared the mRNA expression of several pro-inflammatory mediators, including TNF- α, IL-10, and ICAM-1, before and after blocking visfatin expression with siRNA (Fig. 5A). When GBECs were treated with LPS, these pro-inflammatory mediators were significantly increased (P < 0.05). However, blocking the expression of visfatin with siRNA significantly reduced the expression of these pro-inflammatory mediators (P < 0.05), suggesting that visfatin plays an essential role in increasing the expression of these pro-inflammatory mediators.
Finally, we compared the expression of ABCG1 before and after blocking visfatin expression with siRNA (Fig. 5B). When visfatin was not inhibited, GBECs treated with LPS significantly inhibited the expression of ABCG1 (P < 0.005). However, inhibition of visfatin by si-visfatin significantly abrogated the suppressive effects of LPS on the expression of ABCG1 in the GBECs, suggesting that visfatin is significantly involved in the LPS-driven suppression of ABCG1 (P < 0.005). As ABCG1 is an essential mediator of cholesterol transport, it is expected that visfatin could contribute to increased cholesterol accumulation by reducing the expression of ABCG1 and thus, lead to a more severe inflammatory response.
In this study, we investigated whether serum visfatin level could be a prognostic factor for predicting the severity of inflammation in patients with acute cholecystitis. Acute cholecystitis patients exhibited higher mRNA levels of visfatin in PBMCs, higher serum visfatin levels, and higher protein levels of visfatin in the gallbladder than the chronic cholecystitis patients. We also observed that the mRNA expression of visfatin was increased in the shortest amount of time compared to other pro-inflammatory mediators such as IL-10, TNF-α, IL-6, ICAM-1, and VCAM-1 in the in vitro model of acute cholecystitis. In the in vitro model of acute cholecystitis, when visfatin was not inhibited, the expression of ABCG1 was suppressed in the LPS-treated GBECs. On the other hand, when visfatin was inhibited, the suppressive effects of LPS on the expression of ABCG1 in GBECs were disrupted, suggesting that visfatin is significantly involved in the LPS-driven suppression of ABCG1. In summary, visfatin is a pro-inflammatory mediator that is upregulated during acute cholecystitis and is expected to be increased within a short time after inflammation. Therefore, the serum levels of visfatin could be an effective predictor of the severity of inflammation in the patients with acute cholecystitis.
Our study showed that the patients with acute cholecystitis exhibited higher mRNA levels of visfatin in PBMCs and higher serum levels of visfatin. Visfatin is mainly produced in the adipose tissue and PBMCs. Specifically, visfatin can be considered as an “adipokine” secreted from subcutaneous and visceral adipose tissues as well as a “hormone” released from the PBMCs, such as B cells, T cells, macrophages, and neutrophils. The released visfatin not only acts as a potent pro-inflammatory agent itself, but also stimulates the production of numerous pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-10, and IL-6 [1012]. Visfatin can upregulate the expression of IL-6, IL-8, TNF-α, and MMP-9 in monocytes through the p38-MAPK signaling pathway [19]. Visfatin was also found to increase the number of macrophages, which are major sources of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [20]. Taken together, visfatin can be considered as a central mediator of acute inflammatory response.
The upregulation of visfatin is observed in numerous inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, psoriasis, acute lung injury, and sepsis [10141516]. However, till date, few studies have been conducted elucidating the role of visfatin in cholecystitis. Xie et al. [4] reported that elevated plasma visfatin levels correlated with the conversion rate of laparoscopic cholecystectomy to open surgery in patients with acute cholecystitis. They analyzed 146 patients with acute cholecystitis along with equal number of healthy controls, and identified elevated plasma levels of visfatin as one of the independent factors for predicting the open conversion during laparoscopic cholecystectomy. In another study, patients with gallstones, regardless of the type, were found to have the significantly higher serum levels of visfatin than the control patients [21]. Furthermore, the correlation between higher visfatin levels and the cholesterol gallstones was more significant in obese patients (body mass index ≥ 25 kg/m2) than controls. As the portal vein drains blood to the liver from most of the gastrointestinal tract including visceral adipose tissue, adipokines released from visceral adipose tissue can affect the hepatobiliary system [22]. Thus, visfatin drained into the portal vein might contribute to gallstone formation to a certain degree. This study suggests the possibility of using visfatin as an independent predictor for gallstone formation.
Visfatin can be considered as one of the adipokines, which are soluble mediators that are mainly, but not exclusively, released by adipocytes and exhibit their functions in an autocrine, paracrine or endocrine manner [23]. Besides visfatin, other adipocytokines include TNF-α, IL-6, plasminogen activator inhibitor-1, adiponectin, leptin, resistin, serum amyloid A, angiotensinogen, and monocyte chemoattractant protein-1 [2425]. In general, a significant number of these adipokines are not only released from adipocytes but also from activated macrophages and/or other immune cells. Thus, adipose tissue can be considered as an endocrine or immune organ that is involved in a variety of biological functions, including inflammation, immunity, and endocrine function. Therefore, as obese patients have a larger pool of functional adipose tissues, they are expected to be particularly vulnerable to various diseases associated with inflammation and immunity.
While considering the role of visfatin in the gallbladder disease, one should pay attention to the fact that obesity is the one of the most prominent risk factors for gallstone formation [2627]. It was reported that the patients with obesity and type II diabetes have significantly higher circulating visfatin levels than controls [628], suggesting a close link between visfatin and obesity-related inflammatory diseases, including diabetes, atherosclerosis, dyslipidemia, and cholelithiasis. Obesity is inevitably accompanied by inflammatory response that is characterized by elevated levels of systemic inflammatory cytokines and accumulation of macrophages in adipose tissues [29]. ABCG1 is a member of the ATP-binding cassette transporter superfamily, and is responsible for the cholesterol efflux from macrophages to serum or HDL. Park et al. [30] showed that ABCG1 was repressed by inflammatory insults at least in part via the down-regulation of the PPARγ pathway. Specifically, they showed that LPS significantly decreased the expression of ABCG1 mRNA in mouse peritoneal macrophages. In this study, we showed that visfatin significantly downregulated ABCG1 in GBECs. Downregulated ABCG1 in obese patients can lead to cholesterol accumulation in the tissues, making them more vulnerable to acute inflammation.
In conclusion, we showed that visfatin could be a valuable early prognostic factor for acute cholecystitis. The patients with acute cholecystitis exhibited higher mRNA levels of visfatin in PBMCs, higher serum levels of visfatin, and higher level of visfatin protein in the gallbladder than the chronic cholecystitis patients. In the in vitro model of acute cholecystitis, the mRNA expression of visfatin increased in the shortest time duration compared to other pro-inflammatory mediators, including IL-10, TNF-α, IL-6, ICAM-1, and VCAM-1. Visfatin was also found to inhibit the expression of ABCG1 in the GBECs, which is a cholesterol transporter protein, thereby aggravating the inflammatory response during acute cholecystitis. Therefore, measuring the serum level of visfatin could potentially assist in predicting the natural course of the disease in the patients with acute cholecystitis.
ACKNOWLEDGEMENTS
We would like to thank Hye-Jung Kim for photoshop works that has made the manuscript understood intuitively. We also would like to thank Ji-Hye Park for her data processing and statistical works.
References
1. Trowbridge RL, Rutkowski NK, Shojania KG. Does this pat ient have acute cholecystitis? JAMA. 2003; 289:80–86. PMID: 12503981.
2. Friedman GD. Natural histor y of asymptomat ic and symptomat ic gallstones. Am J Surg. 1993; 165:399–404. PMID: 8480871.
3. Sartelli M, Abu-Zidan FM, Catena F, Griffiths EA, Di Saverio S, Coimbra R, et al. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: a prospective multicentre study (WISS Study). World J Emerg Surg. 2015; 10:61. PMID: 26677396.
4. Xie KG, Teng XP, Zhu SY, Qiu XB, Ye XM, Hong XM. Elevated plasma visfatin levels correlate with conversion of laparoscopic cholecystectomy to open surgery in acute cholecystitis. Peptides. 2014; 60:8–12. PMID: 25086268.
5. Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005; 307:426–430. PMID: 15604363.
6. Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005; 54:2911–2916. PMID: 16186392.
7. de Fougerolles AR, Chi-Rosso G, Bajardi A, Gotwals P, Green CD, Koteliansky VE. Global expression analysis of extracellular matrix-integrin interactions in monocytes. Immunity. 2000; 13:749–758. PMID: 11163191.
8. Liu K, Li Y, Prabhu V, Young L, Becker KG, Munson PJ, et al. Augmentation in expression of activation-induced genes differentiates memory from naive CD4+ T cells and is a molecular mechanism for enhanced cellular response of memory CD4+ T cells. J Immunol. 2001; 166:7335–7344. PMID: 11390484.
9. Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008; 83:804–816. PMID: 18252866.
10. Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007; 178:1748–1758. PMID: 17237424.
11. Stofkova A. Resistin and visfatin: regulators of insulin sensitivity, inflammation and immunity. Endocr Regul. 2010; 44:25–36. PMID: 20151765.
12. Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004; 113:1318–1327. PMID: 15124023.
13. Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, et al. Hormonal regulation of the novel adipocytokine visfatin in 3T3-L1 adipocytes. J Endocrinol. 2005; 185:R1–R8. PMID: 15930160.
14. Koczan D, Guthke R, Thiesen HJ, Ibrahim SM, Kundt G, Krentz H, et al. Gene expression profiling of peripheral blood mononuclear leukocytes from psoriasis patients identifies new immune regulatory molecules. Eur J Dermatol. 2005; 15:251–257. PMID: 16048752.
15. Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006; 54:2084–2095. PMID: 16802343.
16. Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005; 171:361–370. PMID: 15579727.
17. Xiao K, Zou WH, Yang Z, Rehman ZU, Ansari AR, Yuan HR, et al. The role of visfatin on the regulation of inflammation and apoptosis in the spleen of LPS-treated rats. Cell Tissue Res. 2015; 359:605–618. PMID: 25358398.
18. Kobayashi K, Kan M, Yamane I, Ishii M, Toyota T. Primary culture of human gallbladder epithelial cells. Gastroenterol Jpn. 1991; 26:363–369. PMID: 1832405.
19. Romagnolo DF, Davis CD, Milner JA. Phytoalexins in cancer prevention. Front Biosci (Landmark Ed). 2012; 17:2035–2058. PMID: 22652763.
20. Traves PG, Luque A, Hortelano S. Macrophages, inflammation, and tumor suppressors: ARF, a new player in the game. Mediators Inflamm. 2012; 2012:568783. PMID: 23316105.
21. Wang SN, Yeh YT, Wang ST, Chuang SC, Wang CL, Yu ML, et al. Visfatin--a proinflammatory adipokine-in gallstone disease. Am J Surg. 2010; 199:459–465. PMID: 19427623.
22. Arner P. Visfatin--a true or false trail to type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006; 91:28–30. PMID: 16401830.
23. Tilg H, Moschen AR. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci (Lond). 2008; 114:275–288. PMID: 18194136.
24. Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006; 354:2552–2563. PMID: 16775236.
25. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115:1111–1119. PMID: 15864338.
26. Bonfrate L, Wang DQ, Garruti G, Portincasa P. Obesity and the risk and prognosis of gallstone disease and pancreatitis. Best Pract Res Clin Gastroenterol. 2014; 28:623–635. PMID: 25194180.
27. Shiffman ML, Sugerman HJ, Kellum JH, Brewer WH, Moore EW. Gallstones in patients with morbid obesity. Relationship to body weight, weight loss and gallbladder bile cholesterol solubility. Int J Obes Relat Metab Disord. 1993; 17:153–158. PMID: 8385075.
28. Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006; 91:295–299. PMID: 16234302.
29. Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley DE, et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004; 14:589–600. PMID: 15186624.
30. Park Y, Pham TX, Lee J. Lipopolysaccharide represses the expression of ATP-binding cassette transporter G1 and scavenger receptor class B, type I in murine macrophages. Inflamm Res. 2012; 61:465–472. PMID: 22240665.
Fig. 1
Comparison of the expression levels of pro-inflammatory mediators in the blood samples. (A) Real-time polymerase chain reaction showing the differences between the mRNA expression levels of pro-inflammatory mediators in the peripheral blood mononuclear cells (PBMCs) of the patients with chronic and acute cholecystitis. The mRNA levels of the pro-inflammatory markers were significantly higher in the PBMCs of patients with acute cholecystitis than in the chronic cholecystitis patients. Out of all the markers, resistin and visfatin showed the higher increase compared to the expression levels of ICAM-1 and VCAM-1. (B) Enzyme-linked immunosorbent assay results demonstrating the serum levels of pro-inflammatory mediators. The serum levels of the pro-inflammatory markers were significantly higher in acute cholecystitis patients with in chronic cholecystitis patients. Serum levels of visfatin were significantly higher than those of other pro-inflammatory mediators (P < 0.05). Values are presented as mean ± standard deviation of 3 independent experiments. ICAM-1, intracellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; IL, interleukin. *P < 0.05.
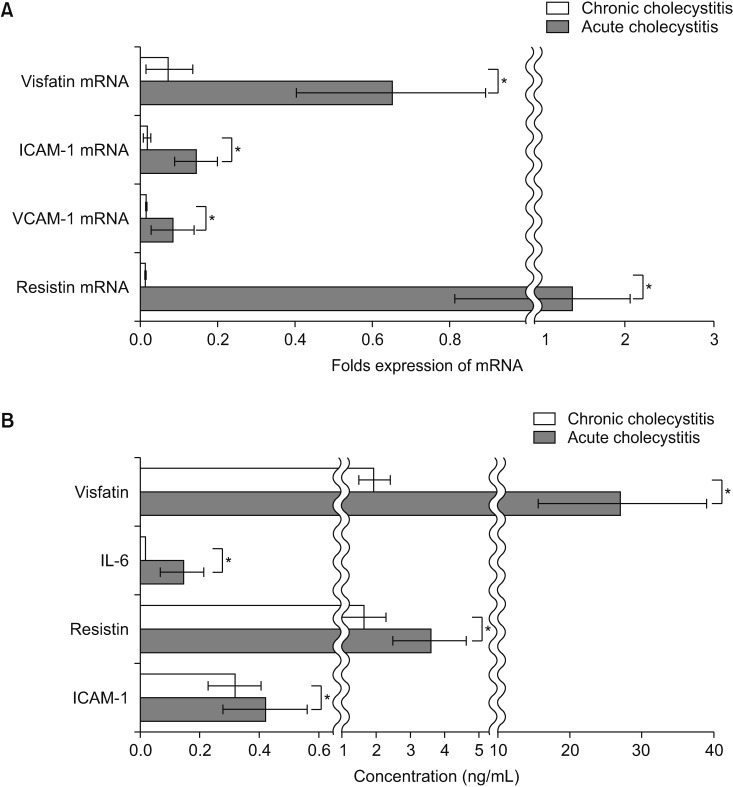
Fig. 2
Pro-inflammatory mediators expressed in the gallbladder specimens. We obtained 2 areas of grossly inflamed and noninflamed gallbladder tissues, respectively, from patients with acute cholecystitis and chronic cholecystitis. (A) In the gallbladder tissues with acute cholecystitis, the expression levels of visfatin and F4/80 were significantly higher in grossly inflamed tissues than in grossly non-inflamed tissues. (B) Relative densities of pro-inflammatory markers in each group. Values are presented as mean ± standard deviation of 3 independent experiments. *P < 0.05.
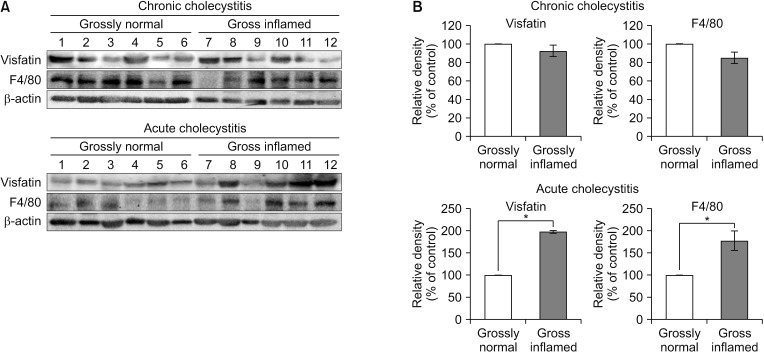
Fig. 3
Comparison of immunohistochemistry of the gallbladder specimens with or without acute cholecystitis. Immunohistochemical staining showed that immunoreactive areas for pro-inflammatory mediators, including visfatin, CD68, ICAM-1, and VCAM-1, were significantly higher in the gallbladder specimens, especially grossly inflamed tissues, of the patients with acute cholecystitis (A) than those of the patients with chronic cholecystitis (B). The percentages of immunoreactive areas were measured using the ImageJ software (National Institute Health, Bethesda, MD, USA) and were expressed as values relative to those in normal gallbladder. Values are presented as mean ± standard deviation of 3 independent experiments. ICAM-1, intracellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1. *P < 0.05.

Fig. 4
Changes of mRNA expression of pro-inflammatory mediators over time in the in vitro model of acute cholecystitis. (A) Determination of LPS concentration for generating in vivo model of acute cholecystitis. The most pro-inflammatory mediators significantly increased when the LPS of concentration above 25-ng/mL concentration was added. We thus use the 25-ng/mL LPS to generate the in vitro model of acute cholecystitis. (B) Real-time polymerase chain reaction showing the time-dependent differences in the levels of these pro-inflammatory mediators in the in vitro model of cholecystitis. The mRNA expression of visfatin was increased in the shortest time span compared to those of the other pro-inflammatory mediators. Values are presented as mean ± standard deviation of 3 independent experiments. ICAM-1, intracellular adhesion molecule-1; LPS, lypopolysaccharide; IL, interleukin; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1. *P < 0.05.
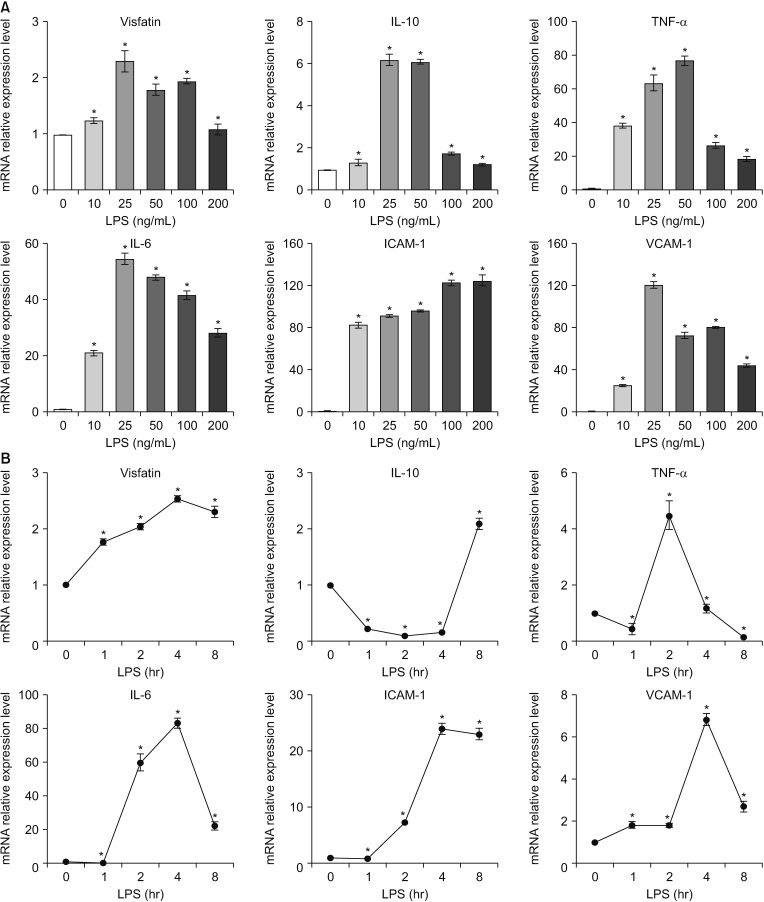
Fig. 5
Determination of the role of visfatin during acute cholecystitis. (A) Real-time polymerase chain reaction comparing the mRNA expression of the pro-inflammatory mediators, including TNF-α, IL-10, and ICAM-1, before after blocking visfatin expression with siRNA. When gallbladder epithelial cells (GBECs) were treated with LPS, these pro-inflammatory mediators were significantly increased (P < 0.05). However, blocking the expression of visfatin with siRNA significantly reduced the expression of these pro-inflammatory mediators. (B) Western blot analysis comparing the expression of ABCG1 before and after blocking visfatin expression with siRNA. When visfatin was not inhibited, GBECs treated with LPS significantly inhibited the expression of ABCG1. However, inhibition of visfatin by si-visfatin significantly abrogated the suppressive effects of LPS on the expression of ABCG1 in the GBECs. Values are presented as mean ± standard deviation of 3 independent experiments. Ct, control; siCt, negative control siRNA; ICAM-1, intracellular adhesion molecule-1; LPS, lypopolysaccharide; SRA, scavenger receptor A; TNF-α, tumor necrosis factor-α; IL, interleukin. *P < 0.05.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download