Abstract
Purpose
The aim of this study was to analyze the effects of reduced fasting time on postoperative recovery in patients who underwent totally laparoscopic distal gastrectomy (TLDG).
Methods
This retrospective study included 347 patients who underwent TLDG. Patients were divided into 2 groups: reduced fasting time group (n = 139) and conventional feeding group (n = 208). We compared the total hospital cost and recovery parameters, such as postoperative complications, mean hospital stay, day of first flatus, initiation of soft diet, and serum CRP levels, between the 2 groups.
Results
The reduced fasting time group had a lower total hospital cost (P < 0.001) than the conventional feeding group. Regarding postoperative complications, there was no significant difference between the 2 groups (P = 0.085). Patients in the reduced fasting time group had a significantly shorter duration of mean hospital stay (P < 0.001), an earlier first flatus (P = 0.002), an earlier initiation of soft diet (P < 0.001), and lower level of serum CRP concentration (day of surgery, P = 0.036; postoperative days 2, 5, and 7, P = 0.01, 0.009, and 0.012, respectively) than patients in the conventional feeding group.
Go to : 
Radical curative gastrectomy and effective bowel reconstruction have been recognized as standard treatments for gastric cancer. The fundamental goals of these treatments are to restore intestinal transit and to provide good nutritional conditions to improve quality of life (QOL) after gastrectomy [1]. In addition, as the prognosis of gastric cancer has improved with the development of surgical techniques and establishment of the national cancer screening program in Korea, improvement of QOL after gastrectomy is becoming increasingly important not only in terms of surgical technique but also in perioperative management. Therefore, early recovery after gastrectomy can have a substantial impact on QOL and restoration of gastrointestinal function; indeed, fast dietary rehabilitation can play an important role in early recovery. Traditionally, fasting, including avoiding food consumption 6–8 hours before elective surgical procedures requiring general anesthesia, was recommended to prevent pulmonary aspiration [23]. Moreover, in conventional treatments of gastric cancer, many surgeons advocated that early oral feeding after gastrectomy could increase the risk of postoperative ileus and more severe anastomotic tension, and these effects could cause issues related to anastomosis, such as leakage or disruption; therefore, total oral intake restriction period was sometimes delayed up to 3–4 days or more [45]. However, increased duration of perioperative fasting can result in increased inflammatory response to surgery, postoperative fluid overload, increased catabolism, and delayed recovery of bowel function [6]. Ultimately, these harmful effects exacerbate malnutrition, increase the incidence of postoperative complications, and lead to poor prognosis.
Recent papers have demonstrated opposing points of views regarding traditional perioperative oral feeding concepts. Some authors reported that drinking carbohydrate-rich fluids 2 hours before elective surgery reduces loss of glycogen and insulin resistance and maintains lean body mass. These authors also suggested that it could decrease postoperative thirst and hunger and improve subjective well-being [7]. Several studies reported that early oral feeding after gastrectomy is not associated with increased risk of gastrointestinal-related complications, such as anastomosis leakage or postoperative ileus; instead, it can reduce postoperative morbidity, mortality, and hospital stay [8].
Therefore, in the present study, we focused mainly on investigating the effects of reducing preoperative fasting time and early oral feeding on the postoperative recovery after totally laparoscopic distal gastrectomy (TLDG).
Go to : 
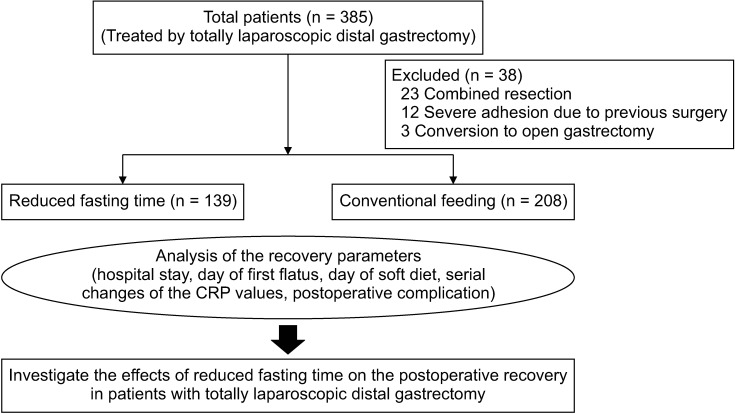
A total of 385 patients with gastric cancer treated using TLDG between January 2016 and December 2018 were included in this study. The same reconstruction method was performed on all patients, which was intracorporeal Billroth II (gastrojejunostomy) with Broun anastomosis (jejunojunostomy). Among 385 patients, 38 patients were excluded as follows: 23 patients who underwent a combined resection of the spleen, liver, or colon; 12 patients with severe adhesion due to previous abdominal surgery; and 3 patients with conversion to open gastrectomy (Fig. 1). The remaining 347 patients were divided into the reduced fasting time group (n = 139) and the conventional feeding group (n = 208).
The schedule for reduced fasting time was initially started as a feasibility assessment for the application of clinical pathway (CP), standardization of perioperative management for patients with gastric cancer from December 2017. All patients were provided with detailed information about reduced fasting time during their management, and those patients who submitted their informed consent were enrolled in the reduced fasting time group.
Data on clinical characteristics, total hospital cost, and postoperative recovery parameters were compared between the 2 groups. The definition of comorbidity in this study was that patients had a history of taking medical treatments for other diseases such as hypertension, diabetic mellitus, cardiovascular disease, cerebrovascular disease, liver disease, and renal disease. Postoperative recovery parameters including signs of a return to normal bowel function, such as the day of first flatus and the day of soft diet initiation, and data regarding mean hospital stay, serum CRP levels, postoperative complications, and readmission rate were collected to assess the impact of reduced fasting time on postoperative recovery. Postoperative complications were accessed in accordance with the Clavien-Dindo classification system, and grade II or higher, which occurred during hospitalization or within 30 days after surgery, was considered complications [9]. All patients were permitted to ingest normal meals until the day before surgery and underwent the same surgical procedure as TLDG.
It was suggested that patients should be discharged if they had no discomfort after 3 soft diet meals, normal passage of gas and stool, no laboratory or clinical signs of complication, and if they were willing to return home.
This study was approved by the International Review Board of Pusan National University Yangsan Hospital (05-2020-128), and a waiver of informed consent was also approved.
Normal meals were served until the day before surgery and carbohydrate-rich drinks were supplied until 2 hours before TLDG. A small amount of water and carbohydrate-rich drinks were provided from 6 hours after TLDG to postoperative day (POD) 1. The patients were advised to have a clear liquid diet on POD 2, a full liquid diet on POD 3, and a soft diet on POD 4 if tolerated without abdominal discomfort or nausea and vomiting. If a patient had difficulty progressing to oral feeding, their diet schedule was postponed by about 1 day. The dietary schedule was designed to keep the total perioperative fasting time under 12 hours. Patients received 30 mL/kg/day of intravenous fluid administration until POD 4; the amount was limited to avoid fluid overload. Hospital discharge was recommended on POD 6 when it was determined that the patient could maintain their diet regimen.
Normal meals were supplied until the day before TLDG, but they were required to fast at least 8 hours before general anesthesia. Water intake was allowed on POD 2, and once postoperative ileus was resolved based on plain X-ray, or after the first flatus, a clear liquid diet was provided from POD 3 or POD 4. Subsequently, according to the conventional regimen, patients were supplied with a full liquid diet and then a soft diet every other day when the surgeon considered that the oral intake was sufficient, and when patients did not feel discomfort after initiating the diet. Hospital discharge was recommended between POD 7 and POD 8 if the patient has no discomfort after 3 soft diet meals.
The chi-square test or Fisher exact test was used to compare the differences in quantitative data, whereas the Student t-test was used to compare the differences in measured data. The change in postoperative serum CRP levels over time was analyzed using a repeated-measures analysis of variance (ANOVA). The significance level of 3 pairwise comparisons at each time point was adjusted by the Bonferroni procedure to account for multiple testing. The level of significance was set at a P-value of <0.05 in all statistical analyses. All statistics were undertaken with IBM SPSS Statistics ver. 21.0 software (IBM Corp., Armonk, NY, USA).
Go to : 
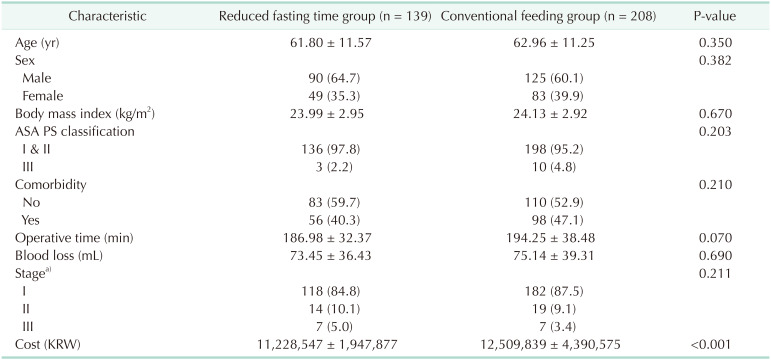
The clinical characteristics of the 2 groups are presented in Table 1. There were no significant differences between the groups regarding age, sex, body mass index, American Society of Anesthesiologists (ASA) physical status, presence of comorbidity, operative time, blood loss, and pathological stage (all P > 0.05). Total hospital cost was lower in the reduced fasting time group than in the conventional feeding group (11,228,547 ± 1,947,877 Korean Won vs. 12,509,839 ± 4,390,575 Korean Won, P < 0.001).
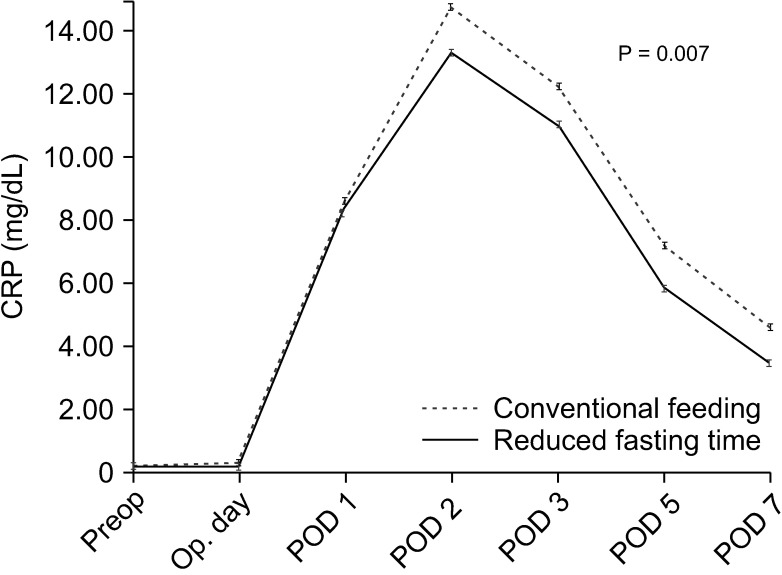
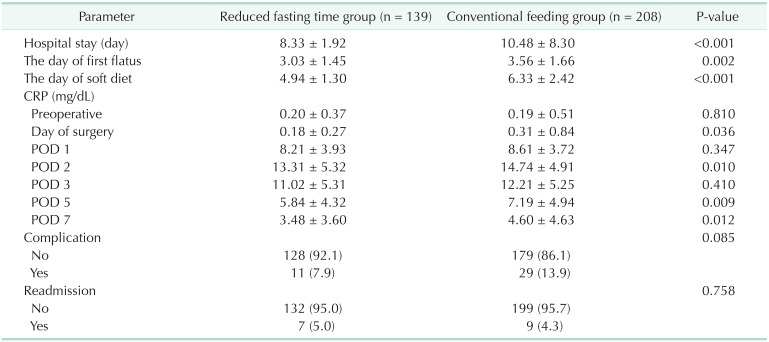
Table 2 summarizes recovery parameters in the 2 groups. The length of hospital stay, the day of first flatus, and the day of initiation of soft diet were shorter in the reduced fasting time group than in the conventional feeding group (P < 0.001, P = 0.002, and P < 0.001, respectively). On the day of surgery, POD 2, POD 5, and POD 7, the mean serum levels of CRP in the reduced fasting time group were significantly lower than those in the conventional feeding group (P = 0.036, P = 0.010, P = 0.009, and P = 0.012, respectively). Postoperative daily changes in serum levels of CRP over time are shown in Fig. 2. The reduced fasting time group had lower CRP concentrations than the conventional feeding group during all postoperative periods. A peak CRP concentration was observed on POD 2 in both groups, but there was a clearly lower CRP concentration in the reduced fasting time group (P < 0.007). Postoperative complications and readmission rates were similar between the 2 groups (all P > 0.05).
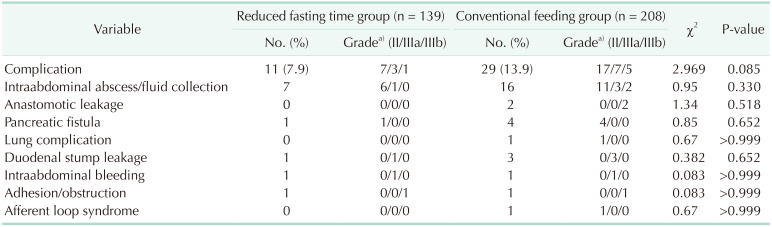
Table 3 shows complications among the reduced fasting time group and conventional feeding group. The incidence of postoperative complications was 7.9% (11/139) in the reduced fasting time group and 13.9% (29/208) in the conventional feeding group, and there were no significant differences between the groups (P = 0.085). In both groups, an intraabdominal abscess, or fluid collection, was the most frequent complication (7/139 [5.0%], 16/208 [7.7%], respectively; P = 0.330). Two patients in the conventional feeding group had an anastomotic leak and fully recovered after reoperation.
Go to : 
An increased incidence in early gastric cancer and widespread use of laparoscopy have contributed to a growing interest in improving the QOL of patients after gastrectomy [10]. Indeed, enhanced recovery after surgery (ERAS) protocols have been applied in various surgical fields, with several studies investigating the applicability of the ERAS protocols in gastric cancer [11]. These ERAS protocols include preoperative carbohydrate-rich drink 2 hours before surgery, avoiding fluid overload, and early initiation of oral feeding to minimize fasting time, and it is considered one of the most important multimodal strategies to enhance postoperative recovery [12].
Considering that elective surgery is a type of planned trauma, this surgical stress can lead to increased insulin resistance, catabolic effects, and loss of lean body mass. In addition, most endogenous glucose production is decreased after fasting and glycogen stores in the liver and muscles are depleted after 48 hours of fasting, and this response can be accelerated in the state of insulin resistance [13]. Indeed, total fasting time can be prolonged up to 3–4 days or more in conventional perioperative treatment of patients with gastric cancer. Meanwhile, insulin resistance is known to begin preoperatively when fasting before surgery. Therefore, prolonged perioperative fasting time can increase insulin resistance, and may impact the metabolic response and increase catabolism, thereby affecting postoperative recovery [14].
This study was designed as a feasibility assessment for the application of CP to patients with TLDG in our institution. Therefore, its core elements include preoperative carbohydrate-rich drink 2 hours before surgery for preoperative short fasting, prevention of fluid overload, and early oral feeding, including intake of water and carbohydrate-rich drink from 6 hours postoperatively to POD 1, a clear liquid diet on POD 2, a full liquid on POD 3, and a soft diet daily starting on POD 4 in 3 steps (clear liquid–full liquid–soft diet). This schedule was intended to minimize the total fasting time of the perioperative period to less than 12 hours.
Recently, Goyal et al. [15] have reported that gastric emptying time of solid food is about 4–6 hours and that of a liquid form is 2 hours. Likewise, the rationale for preoperative short fasting was described in the ASA Committee on standards and practice parameters [16]. Based on the ASA Committee recommendation, it is appropriate to fast from intake of clear liquids at least 2 hours, a light meal such as toast at least 6 hours, and a meal like fried or fatty foods at least 8 hours, before elective procedures requiring general anesthesia, regional anesthesia, or sedation/analgesia [16]. Some authors report that preoperative carbohydrate-rich drink 2 hours before surgery can increase reserves of liver glycogen by 44%, and may attenuate the development of postoperative insulin resistance, and reduce acute phase response, such as catabolism, after surgery [17]. Furthermore, it can decrease the postoperative feelings of thirst and hunger and improve subjective well-being [718].
Meanwhile, early initiation of oral feeding can lead to additional improvement in surgical outcomes. The introduction of this concept in the field of upper gastrointestinal surgery was suggested by Gabor et al. [19] and they demonstrated that it is safe to begin enteral nutrition at 6 hours postoperatively. Schroeder et al. [20] reported that early oral feeding can improve wound healing, which is particularly relevant to the integrity of the intestinal anastomosis, muscle function, insulin resistance, and reduced sepsis. As previously mentioned, over the past decade, there have been several studies on ERAS in gastric cancer treatment, which advocated early oral feeding in postoperative recovery [11]. These studies reported that the benefits of early oral feeding include facilitating a more rapid postoperative recovery, significant reduction in postoperative hospital stay, and decreased cost without increasing the prevalence of complications or readmission rate.
Table 2 summarizes the postoperative recovery parameters. As with previous studies, there were significant reductions in the time until the first flatus, initiation of a soft diet, and length of hospital stay in the reduced fasting time group. These findings indicate that early oral feeding can enhance early adaptation to postoperative oral intake, and accelerate postoperative recovery by facilitating rehabilitation of bowel function.
As previously mentioned, surgery is a kind of planned trauma; therefore, inflammation is a normal process of recovery after trauma. However, exaggerated inflammatory response and surgical stress may be associated with the incidence of postoperative complications [21]. Serum levels of CRP were used as indicators of the postoperative inflammatory response; therefore, they are useful serum markers for reflecting the severity of surgery-induced stress and can be used to evaluate the response to treatment and the degree of recovery after surgery [22]. In this study, Fig. 2 and Table 2 show postoperative daily changes in serum levels of CRP over time, and the reduced fasting time group had lower serum levels of CRP than the conventional feeding group during all postoperative periods. Based on these data, it is presumed that reduced fasting time in patients undergoing TLDG can reduce surgery-induced stress, improve the postoperative inflammatory response, and enhance the postoperative recovery of patients. These results are consistent with previous studies [11].
One of the most important matters in reduced fasting time is whether it is related to postoperative complications and readmission or not. And the surgical outcomes of perioperative management can also be assessed by them. In the present study, Table 2 and 3 show that reduced fasting time in patients with TLDG did not significantly increase the incidence of postoperative complications and readmission, especially gastrointestinal-related complications, such as anastomosis leakage, intraabdominal bleeding, and intestinal obstruction. These results are consistent with past studies, namely that early oral feeding improves wound healing and anastomotic strength in the intestines [20]. Therefore, it is unreasonable to restrict oral intake for fear of anastomosis-related complications and postoperative ileus in conventional perioperative management after TLDG.
Regarding hospital costs, our results indicate that reduced perioperative fasting time is cost-effective. The total hospital cost in the reduced fasting time group was significantly lower than that in the conventional feeding group; this result may be associated with the rapid postoperative recovery, reduced hospital stay, and a relative decrease of parenteral nutrition support due to avoidance of fluid overload.
These advantages for early oral feeding are due to the fact that the gut plays a pivotal role in maintaining nutritional status and regulating the immune system, and its background is reported by the following past studies. First, the time required for reinitiation of peristalsis of the small bowel is about 6–12 hours after surgery, and it is known to maintain absorption capacity despite the absence of peristalsis [23]. Therefore, luminal nutrients can be effectively transmitted and absorbed even in early oral feeding after surgery. Second, early oral feeding is itself a physiological feeding channel, so it preserves intestinal mucosal integrity, such as maintaining function and morphology, reducing the displacement of intestinal flora, and preventing increased mucosal permeability, thereby preserving natural immunity and barrier function [24]. Third, early oral feeding is related to reduction of hypermetabolic state, improvement of nitrogen balance, and enhancement of immune function [2425]. Surgical stress can cause a reduction in the oxygen tension of the gastrointestinal tract as a physiologic postoperative response [26]. However, these effects can be prolonged if the gut is in a state of resting, such as fasting or parenteral nutrition. Nutrients from early oral feeding directly stimulate epithelial cell metabolism and turnover and activate gastrointestinal hormone secretion to improve intestinal blood flow and gastrointestinal motility [27]. These effects lead to a significant rapid recovery of oxygen tension, which increases the systemic and local immune response [28]. Fourth, as mentioned above, prolonged perioperative fasting time can increase insulin resistance [14]. Early oral feeding is the best way to reduce postoperative insulin resistance by increasing the delivery of nutrients like glucose, directly providing energy supply, and inducing prompt insulin response [29].
Therefore, it is recommended that oral feeding begin as soon as possible after surgery. With this in mind, we designed this study with a schedule to start oral feeding from 6 hours postoperatively and reduced total fasting time in the perioperative period to less than 12 hours.
Since this was a retrospective, nonrandomized, and single-center study, there may be selection bias for patients, despite comparable clinical characteristics between the 2 groups. Thus, large-scale multicenter, randomized, and controlled trials should be carried out to generalize the efficacy of reduced perioperative fasting time.
In terms of QOL, the fast recovery of dietary rehabilitation after gastrointestinal surgery, especially after gastrectomy, is a very important surgical achievement. Indeed, reduced fasting time in patients with TLDG can enhance postoperative recovery, reduce hospital stay, and decrease medical cost without increasing the prevalence of complications or readmission rates. Therefore, this study contributes to this field by corroborating results of previous studies that demonstrate the benefit of reduced fasting time in the perioperative period.
Go to : 
References
1. Park KB, Yu B, Park JY, Kwon OK, Yu W. Impact of body mass index on quality of life after distal gastrectomy for gastric cancer. Ann Surg Treat Res. 2019; 96:250–258. PMID: 31073515.
2. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993; 78:56–62. PMID: 8424572.
3. de Aguilar-Nascimento JE, Dock-Nascimento DB. Reducing preoperative fasting time: a trend based on evidence. World J Gastrointest Surg. 2010; 2:57–60. PMID: 21160851.
4. Ahn HS, Yook JH, Park CH, Park YK, Yu W, Lee MS, et al. General perioperative management of gastric cancer patients at high-volume centers. Gastric Cancer. 2011; 14:178–182. PMID: 21373856.
5. Lassen K, Dejong CH, Ljungqvist O, Fearon K, Andersen J, Hannemann P, et al. Nutritional support and oral intake after gastric resection in five northern European countries. Dig Surg. 2005; 22:346–352. PMID: 16293965.
6. Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002; 359:1812–1818. PMID: 12044376.
7. Li L, Wang Z, Ying X, Tian J, Sun T, Yi K, et al. Preoperative carbohydrate loading for elective surgery: a systematic review and meta-analysis. Surg Today. 2012; 42:613–624. PMID: 22581289.
8. Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. 2009; 13:569–575. PMID: 18629592.
9. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250:187–196. PMID: 19638912.
10. Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008; 248:721–727. PMID: 18948798.
11. Li MZ, Wu WH, Li L, Zhou XF, Zhu HL, Li JF, et al. Is ERAS effective and safe in laparoscopic gastrectomy for gastric carcinoma? A meta-analysis. World J Surg Oncol. 2018; 16:17. PMID: 29373978.
12. Wang D, Kong Y, Zhong B, Zhou X, Zhou Y. Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg. 2010; 14:620–627. PMID: 20108171.
13. Ljungqvist O, Jonathan E. Rhoads lecture 2011: insulin resistance and enhanced recovery after surgery. JPEN J Parenter Enteral Nutr. 2012; 36:389–398. PMID: 22577121.
14. Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999; 2:69–78. PMID: 10453333.
15. Goyal RK, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil. 2019; 31:e13546. PMID: 30740834.
16. American Society of Anesthesiologists Committee. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011; 114:495–511. PMID: 21307770.
17. Soop M, Nygren J, Myrenfors P, Thorell A, Ljungqvist O. Preoperative oral carbohydrate treatment attenuates immediate postoperative insulin resistance. Am J Physiol Endocrinol Metab. 2001; 280:E576–E583. PMID: 11254464.
18. Jeong O, Ryu SY, Jung MR, Choi WW, Park YK. The safety and feasibility of early postoperative oral nutrition on the first postoperative day after gastrectomy for gastric carcinoma. Gastric Cancer. 2014; 17:324–331. PMID: 23771588.
19. Gabor S, Renner H, Matzi V, Ratzenhofer B, Lindenmann J, Sankin O, et al. Early enteral feeding compared with parenteral nutrition after oesophageal or oesophagogastric resection and reconstruction. Br J Nutr. 2005; 93:509–513. PMID: 15946413.
20. Schroeder D, Gillanders L, Mahr K, Hill GL. Effects of immediate postoperative enteral nutrition on body composition, muscle function, and wound healing. JPEN J Parenter Enteral Nutr. 1991; 15:376–383. PMID: 1910100.
21. Sato N, Koeda K, Ikeda K, Kimura Y, Aoki K, Iwaya T, et al. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg. 2002; 236:184–190. PMID: 12170023.
22. Lee SH, Kim KH, Choi CW, Kim SJ, Kim DH, Choi CI, et al. Reduction rate of C-reactive protein as an early predictor of postoperative complications and a reliable discharge indicator after gastrectomy for gastric cancer. Ann Surg Treat Res. 2019; 97:65–73. PMID: 31384611.
23. Hoover HC Jr, Ryan JA, Anderson EJ, Fischer JE. Nutritional benefits of immediate postoperative jejunal feeding of an elemental diet. Am J Surg. 1980; 139:153–159. PMID: 6766049.
24. Heyland DK, Cook DJ, Guyatt GH. Enteral nutrition in the critically ill patient: a critical review of the evidence. Intensive Care Med. 1993; 19:435–442. PMID: 8294625.
25. Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001; 29:242–248. PMID: 11246300.
26. Beier-Holgersen R, Brandstrup B. Influence of early postoperative enteral nutrition versus placebo on cell-mediated immunity, as measured with the Multitest CMI. Scand J Gastroenterol. 1999; 34:98–102. PMID: 10048740.
27. Ljungqvist O, Nygren J, Thorell A. Insulin resistance and elective surgery. Surgery. 2000; 128:757–760. PMID: 11056438.
28. Johnson CD, Kudsk KA. Nutrition and intestinal mucosal immunity. Clin Nutr. 1999; 18:337–344. PMID: 10634917.
29. Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med. 1995; 152(5 Pt 1):1545–1548. PMID: 7582291.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download