Abstract
Purpose
Tenofovir disoproxil fumarate is accepted as an effective and tolerable drug for treatment of HBV, similar to entecavir. However, there are some concerns about the nephrotoxicity of tenofovir. The aim of this study is to compare the renal-function change of liver recipients who received tenofovir or entecavir for HBV.
Methods
Among 468 patients with HBV who underwent liver transplantation at Samsung Medical Center between January 2008 and December 2015, the patients treated with tenofovir (n = 39) or entecavir (n = 429) were reviewed retrospectively. Baseline characteristics and renal-function change after 1 month, 1 year, and 2 years were compared. Propensity-score matching was performed for 37 patients using tenofovir and 132 patients using entecavir. We also analyzed risk factors of renal dysfunction.
Results
Age, preoperative creatinine, estimated glomerular filtration rate (e-GFR), and hepatic encephalopathy score showed statistical difference between the tenofovir and entecavir groups. The proportion of patients with ‘decreased renal function (e-GFR < 60 mL/min/1.73 m2)’ was higher in the tenofovir group than in the entecavir group (33.3% vs. 12.4% at postoperative one year, P < 0.005). After propensity-score matching, there was no statistical difference in preoperative characteristics. Postoperative 1-, 2-, and 3-year e-GFR and creatinine showed no statistical difference in either group. On multivariate analysis, only preoperative high e-GFR showed a protective effect on renal-function change (odds ratio, 0.97; P < 0.001), and there was no aggravating factor.
Liver transplantation (LT) is the most effective treatment for end-stage liver disease (ESLD). It is also used for treating hepatocellular carcinoma (HCC) when liver cirrhosis is combined or cancer repeatedly recurs after previous resection. Fortunately, postoperative outcomes are gradually improving because of development of surgical skills, medical treatment, and supporting resources. Recent study showed 74.7% patient survival at 5-year in 1,000 cases of LT in single center [1].
In the era of viral suppression, many medical treatments have been developed for treating HBV, which is one of the most common causes of ESLD leading to LT. Nucleotide analogue polymerase inhibitors, including lamivudine, adefovir, entecavir (ETV), telbivudine, and tenofovir, are currently available for treating HBV [2]. Tenofovir disoproxil fumarate (TDF), like ETV, is accepted as an effective and tolerable drug and is recommended as treatment of choice for HBV [2]. TDF and ETV showed high rates of HBV-DNA suppression, HBeAg seroconversion, and ALT normalization while maintaining renal function. However, some studies show renal toxicity of TDF in long-term follow-up study [3].
LT itself is a risk factor for renal dysfunction, especially when patients have comorbidities, such as diabetes mellitus (DM), coronary artery disease, or primary nonfunction of the graft [4]. Therefore, in liver-transplanted patients, it is important to decrease the burden on kidneys when calcineurin inhibitors (CNI) for immunosuppression show possible nephrotoxicity.
However, there are not currently enough studies about renal toxicity of TDF, especially in the transplantation era. The aim of this study is to compare the renal function of post-LT patients who received TDF or ETV for HBV.
From January 7, 2007 to December 29, 2015, patients with HBV who underwent LT (both living-donor LT [LDLT] and deceased-donor LT [DDLT]) at Samsung Medical Center (Seoul, Republic of Korea) were included. Primary diseases for transplantation include HCC, liver cirrhosis, and acute liver failure. Data were collected for 3 years after operation. HBsAg positivity, HBV-DNA positivity, and continuous use of TDF or ETV for HBV prevention were included. HCV infection, HIV infection, HDV infection, autoimmune hepatitis, previous renal disease such as IgA nephropathy, focal segmental glomerulosclerosis or other chronic renal failure, and death or re-transplantation within 2 years after operation were exclusion criteria. We defined ‘chronic renal failure’ as ‘e-GFR < 60 mL/min/1.73 m2 body surface area for at least 3 months’. Patients with acute renal failure (ARF) with aggravation of e-GFR for less than 3 months were not excluded. The study was approved by IRB in Samsung Medical Center (No. 2019-07-038-001). We received written informed consent from all patients.
Patient baseline characteristics of age, sex, body mass index (BMI), albumin, total bilirubin, international normalized ratio, model for ESLD (MELD) score, and Child-Turcotte-Pugh (CTP) score were measured before operation. Serum ALT, HBeAg, HBsAg, and HBV-DNA levels were also evaluated at baseline. For evaluation of renal-function change, estimated glomerular filtration rate (e-GFR) and serum creatinine level (Cr) were measured preoperatively and every year after operation. Other categorical values, such as hepatic encephalopathy, ascites, Child-Pugh class, hepatorenal syndrome, and combined diseases like hypertension (HTN) or DM, were also assessed and compared. Reoperation associated with LT complication was also analyzed. This operation includes bleeding control, hepatic artery, portal vein or graft vein revision, hepaticojejunostomy due to bile leak, duodenal repair due to perforation and wound closure. Reoperation such as liver resection due to HCC recurrence was not included.
We defined e-GFR level less than 60 mL/min/1.73 m2 as ‘decreased renal function’ depending on the National Kidney Foundation practice guideline [5]. We analyzed patients with decreased renal function at 1 month and 1, 2, and 3 years after LT.
For patients with positive HBsAg, 10,000 U of hepatitis B Ig (HBIG) was injected at anhepatic phase intraoperatively. For patients with positive HBV DNA or HBeAg, 20,000 U was injected until posttransplant 4 weeks. HBIG then was injected once daily until postoperative day 6 (7 doses) and once weekly for the next 3 weeks (total 10 times during the first month). Subsequently, 10,000 U of HBIG was injected every month until 1 year after operation. After 1 year, HBIG injection was based on hepatitis B surface antibody titer. For oral antiviral agent, a patient received tenofovir if she/he did not take antiviral agent before transplantation. If the patient used antiviral agent medication before transplantation, the same agent was used after transplantation. Dose of ETV was 0.5 mg once daily, and dose of tenofovir was 300 mg daily. Both agents were tapered properly if patient creatinine clearance was lower than 50 mL/min.
As the induction agent for transplantation, 20 mg of basiliximab was injected intraoperatively and at postoperative day 4. Patients received triple therapy of CNI (tacrolimus or cyclosporin), mycophenolate mofetil (MMF), and steroid for maintenance of immunosuppression. CNI was started the evening of postoperative day 3, with higher targeted level for 2 weeks to prevent early rejection (10–12 mg/dL for tacrolimus and 200–250 ng/mL for cyclosporin). Dose tapering was performed gradually after the first 2 weeks and reached 3–5 mg/dL (for tacrolimus) or 100–150 ng/mL (for cyclosporin) at 3 months. After 3 months, tacrolimus level was adjusted by case considering pattern of AST. MMF was started the morning of postoperative day 1 at 750 mg twice a day. The steroid methylprednisolone was injected intravenously 500 mg intraoperative and at postoperative day 1, tapered over 1 week, and changed to oral methylprednisolone 16 mg twice a day. This oral dose was gradually tapered and stop at 3 months if the patient showed no rejection. This level was reduced when a patient was at high risk of infection (patient older than 65 years or with underlying disease) or seemed to experience CNI-nephrotoxicity. In such cases, tacrolimus level was reduced by 2–3 mg/dL and cyclosporin by 30–50 ng/mL. When a patient showed infection after transplantation, MMF was stopped until recovery. This policy was equally applied to patients using ETV or tenofovir.
Data were evaluated with the IBM SPSS Statistics, ver. 25.0 (IBM Corp., Armonk, NY, USA) statistical program. We compared baseline characteristics using the Wilcoxon rank sum test for numerical variables and the chi-square or Fisher exact test for categorical values. For comparing patients with decreased renal function between the 2 groups, we used the chi-square test or Fisher exact test. To adjust preoperative values that showed differences between the 2 groups, propensity-score matching was used. We consider data with P < 0.05 to be significantly different.
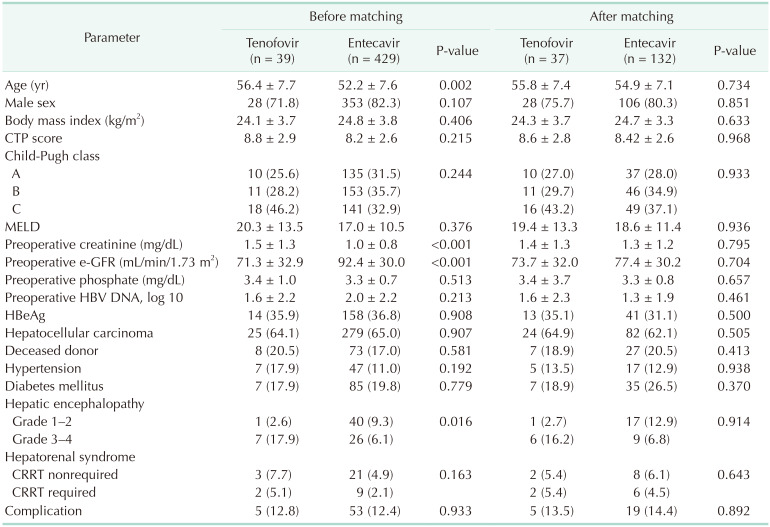
In Samsung Medical Center, 468 patients with HBV underwent LT; of these, 39 were treated with TDF and 429 with ETV. Table 1 shows that the patients of the TDF group were older than those of the ETV group (mean age, 56.36 vs. 52.17 years; P = 0.002). The Cr was higher (1.45 vs. 1.00 mg/dL, P < 0.001) and e-GFR level was lower (71.31 vs. 92.39 mL/min/1.73 m2, P < 0.001) in the TDF group.
Categorical values of type of transplantation (deceased or living donor), proportion of HCC, sex, ascites, Child-Pugh class, and underlying disease (HTN, DM, etc.) showed no statistical difference between the 2 groups, although high-grade (3 or 4) hepatic encephalopathy was more frequent in the TDF group (P = 0.016). Total 62 patients received reoperation due to LT complication without statistical difference between the 2 groups (P = 0.933).
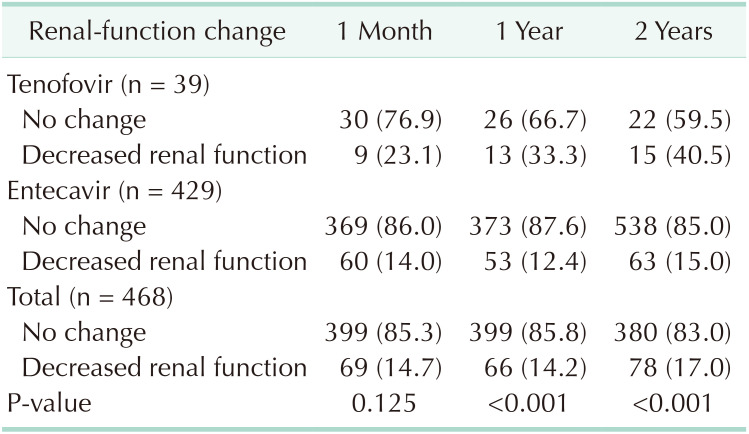
In considering renal-function change, the TDF group showed a higher chance of decreased renal function than did the ETV group (Table 2). The e-GFR was less than 60 mL/min/1.73 m2 in 23.1% of patients with decreased function at one month, 33.3% at one year, and 40.5% at 2 years after operation in the TDF group.
For correcting bias because of the differences of baseline characteristics in the 2 groups, we applied propensity-score matching. The target matching number ratio was 1:4 (TDF:ETV), and matched variables were age, preoperative e-GFR, CTP score, and log 10 value of HBV-DNA titer. After matching, the total sample size was 169 cases, the TDF group was 37, and the ETV group was 132. Some patients in the TDF group were matched to only 1 to 3 patients in the ETV group; thus, the final matching ratio was not exactly 1:4. There was no statistical difference in baseline characteristics between the groups (Table 1).
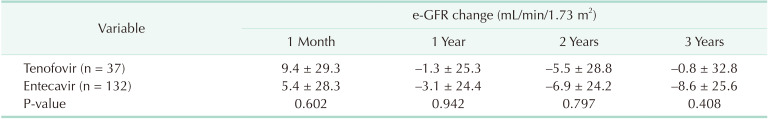
In both groups, e-GFR level showed a slight elevation at one month after transplantation (Table 3) but a slight decrease at 1, 2, and 3 years (−1.28, −5.51, and −0.77 mL/min/1.73 m2 in the TDF group, −3.10, −6.86, and −8.61 mL/min/1.73 m2 in the ETV group, respectively). However, these changes showed no statistical difference (P > 0.05).
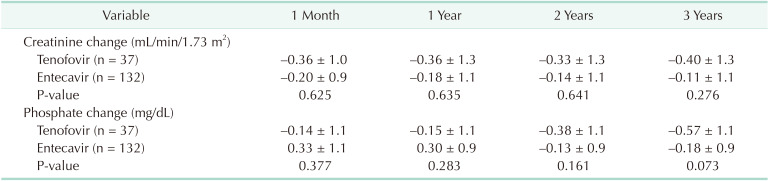
Table 4 shows slight decrease in creatinine after LT without significant difference between the groups (−0.36, −0.33, and −0.40 mg/dL in the TDF group at 1, 2 and 3 years and −0.18, −0.14, and −0.11 mg/dL in the ETV group, respectively). Change of phosphate after LT also showed no noticeable difference between the 2 groups (P > 0.05).
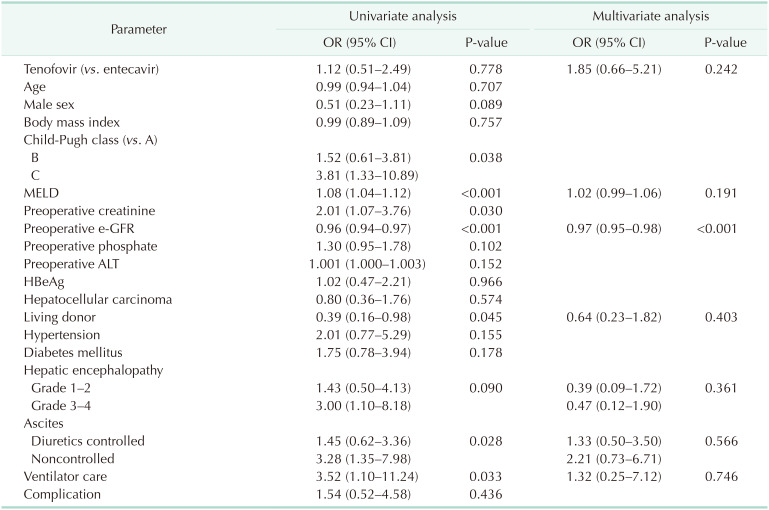
Univariate analysis showed that some factors were associated with high risk for e-GFR decrease (Table 5). High MELD score, deceased-donor LT, noncontrolled ascites, and preoperative ventilator use showed decreased e-GFR after LT (P < 0.05). After matching, 24 cases of reoperation due to complication also did not aggravated e-GFR decrease (odds ratio [OR], 1.54; P = 0.436). These cases include bleeding control, hepatic artery revision, hepatic venous graft revision, superior mesenteric vein thrombectomy, hepaticojejunostomy due to bile leak, duodenal primary repair due to perforation and wound repair (n = 11, 3, 1, 1, 1, 1, and 5, respectively). In contrast, preoperative high e-GFR showed a protective effect from e-GFR decrease in univariate analysis (OR, 0.96; P < 0.001).
In multivariate analysis, no factor significantly aggravated e-GFR change. Donor type, ascites, MELD score, and preoperative ventilator care showed no difference. Only preoperative high e-GFR showed a protective effect on postoperative e-GFR (OR, 0.97; P < 0.001).
Before matching, our study showed a larger proportion of patients with decreased renal function in the TDF group than in the ETV group (33.3% vs. 12.4% after 1 year), perhaps because we defined ‘decreased renal function’ as ‘e-GFR level less than 60 mL/min/1.73 m2’. The baseline e-GFR of the TDF group was much lower than that of the ETV group, very close to 60 mL/min/1.73 m2. Mean age and preoperative Cr were also higher in the TDF group, showing that baseline was different between the groups. For correcting bias, we analyzed data using propensity-score matching and found no statistical difference of e-GFR change or creatinine change between the groups. In multivariate analysis, there was no aggravating risk factor for decreased e-GFR, and preoperative high e-GFR showed a protective effect.
ETV inhibits HBV replication and was approved in 2005 for treatment of naïve HBV patients or lamivudine-resistant patients [6]. It showed a favorable outcome even for lamivudine-refractory patients. TDF was approved originally for HIV infection and then was approved for HBV in 2008 with 300-mg dosing per day (as Viread, Gilead Sciences, Foster City, CA, USA). The American Association for the Study of Liver Diseases had recommended first-line oral antiviral medications as TDF or ETV since 2009 based on 2 double-blind randomized trials showing that TDF shows better outcome than adefovir [7].
In our hospital, TDF has been used since 2013. Originally, the hospital hepatologist used TDF for ETV-resistant HBV patients or patients who had side effects for ETV. Over time, with publication of papers showing its safety and effectiveness, TDF was also used for first-line therapy in newly found HBV. Patients who underwent LT had usually taken the same antiviral agent used before operation when the drug showed no side effects. For patients who did not use an antiviral agent before LT, TDF was prescribed.
Concerns about renal toxicity of nucleotide analogues are common. Gara et al. [3] studied long-term follow-up (mean 7.4 years) of patients using adefovir or tenofovir and showed that 14% of patients developed renal tubular dysfunction that was partially reversible after changing to other antivirals. There is also concern because some HIV patients show tenofovir-induced Fanconi syndrome [8]. However, these side effects were very rare, and there were only a few cases reported [9]. There is also a meta-analysis showing greater renal dysfunction and ARF induced by tenofovir than by ETV in HIV patients [10]. However, that report concludes that the amount of renal dysfunction is very small (mean difference of estimated Cr clearance is 3.92 mL/min; chance of ARF was 0.7%), so it is not appropriate to restrict tenofovir because of concern about renal toxicity.
Some recent studies showed that TDF is better than ETV in outcomes in HCC prevention. Choi et al. [11] analyzed 24,156 patients in the South Korean national cohort group and showed that TDF lowered the risk of HCC siginificantly more than did ETV (hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.59–0.77). This study also used propensity-score matching with a 10,923-pair population cohort showing that TDF lowered the risk of HCC (HR, 0.68; 95% CI, 0.60–0.78; P < 0.05). Another study showed that TDF was better in lowering HCC recurrence than was ETV in patients who underwent liver resection because of HCC (HR, 0.35; 95% CI, 0.33–0.84; P < 0.05) [12].
A new drug, tenofovir alafenamide, was approved in 2016 by the U.S. Food and Drug Administration for HBV and HIV treatment. There are a few reports showing that tenofovir alafenamide is similar in viral suppression and improvement in renal and bone safety compared to TDF [13]. More study about tenofovir alafenamide is expected for HBV patients in the liver transplant era.
The present study had several limitations. First, the number of cases in the TDF group was relatively small because TDF has been used only since 2013 in our hospital. Follow-up duration is also short compared to previously mentioned studies, such as by Gara et al. [3], with a long-term follow-up duration (mean 7.4 years) showing renal dysfunction of nucleotide analogue polymerase inhibitors. Patients with high risk, such as hepatic encephalopathy, were more often tenofovir users, although that was corrected by the propensity-matching maneuver. Second, proteinuria and abnormal sediment in urine are a valuable indicator and aggravating factor for renal parenchymal disease [14]. However, our center did not assess the urinalysis of LT patients serially after transplantation. Urinalysis was tested only at postoperative days 0 to 7 and at 2 weeks and 3 weeks or with clinically suspected renal dysfunction. It would be useful to analyze these parameters in further study.
Not only tenofovir or ETV, but also LT itself is an aggravating factor for renal dysfunction. Weber et al. [15] reviewed some studies showing acute kidney injury occurred with 30% incidence (12%–95%), and chronic kidney disease developed with 22% of 5-year cumulative incidence. These results derive from various causes, such as peritransplant acute tubular necrosis, posttransplant diabetes, or HTN and CNI-related nephrotoxicity. Risk factors of renal dysfunction are known as comorbidities, such as DM, coronary artery disease, or primary nonfunction of the graft [4]. Also, there are also many LT postoperative events that may damage kidneys, such as reoperation, septic or hypovolemic shock. Reoperation of our study showed no significant difference between the 2 groups and did not aggravate e-GFR change. However, we did not compare septic or hypovolemic shock of all patients in this study.
Nephrotoxicity of tenofovir compare to ETV ‘in liver-transplanted’ patients is not studied widely yet. Teperman et al. [16] showed renal safety of tenofovir combined with emtricitabine in liver-transplanted patient in randomized trial. This study showed stable creatinine clearance of 37 patients with combined tenofovir and emtricitabine comparing HBIG withdrawal. While our study showed stable renal function of 37 patients with tenofovir compare to 132 patients with ETV. Jiménez-Pérez et al. [17] studied ETV and/or tenofovir is efficient and safe for HBV treatment in LT patients. Four patients received combined ETV and tenofovir while other 4 patients received only tenofovir showing all of 8 patients had no HBV recurrence with stable renal function which is similar as result of our study. There are some studies showing comparable renal safety between tenofovir and ETV in HBV patients, but these studies only include non-liver-transplanted patients [1819]. However, there is another systematic review showing tenofovir may aggravate renal function compared to ETV in non-LT HBV patients [20]. There are still debates on renal safety of tenofovir and ETV, and this subject of LT era needed more studies. Our study has a meaning in that we directly compared the nephrotoxicity between tenofovir and ETV in relatively large number of LT patients with matching maneuver, showing tenofovir is comparable to ETV regarding renal safety.
In conclusion, TDF can be used safely without additional renal toxicity compared to ETV in liver-transplanted patients.
References
1. Kwak BJ, Kim DG, Han JH, Choi HJ, Bae SH, You YK, et al. Clinical outcome of 1,000 consecutive cases of liver transplantation: a single center experience. Ann Surg Treat Res. 2018; 95:267–277. PMID: 30402445.
2. Sriprayoon T, Mahidol C, Ungtrakul T, Chun-On P, Soonklang K, Pongpun W, et al. Efficacy and safety of entecavir versus tenofovir treatment in chronic hepatitis B patients: a randomized controlled trial. Hepatol Res. 2017; 47:E161–E168. PMID: 27176630.
3. Gara N, Zhao X, Collins MT, Chong WH, Kleiner DE, Jake Liang T, et al. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012; 35:1317–1325. PMID: 22506503.
4. Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003; 9:741–747. PMID: 12827563.
5. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003; 139:137–147. PMID: 12859163.
6. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007; 45:507–539. PMID: 17256718.
7. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009; 50:661–662. PMID: 19714720.
8. Mathew G, Knaus SJ. Acquired Fanconi's syndrome associated with tenofovir therapy. J Gen Intern Med. 2006; 21:C3–C5. PMID: 17026723.
9. Cho H, Cho Y, Cho EJ, Lee JH, Yu SJ, Oh KH, et al. Tenofovir-associated nephrotoxicity in patients with chronic hepatitis B: two cases. Clin Mol Hepatol. 2016; 22:286–291. PMID: 27377911.
10. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010; 51:496–505. PMID: 20673002.
11. Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of hepatocellular carcinoma in patients treated with entecavir vs tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 2019; 5:30–36. PMID: 30267080.
12. Zhang M, Wang D, Liu H, Li H. Tenofovir decrease hepatocellular carcinoma recurrence in chronic hepatitis B patients after liver resection. Infect Agent Cancer. 2018; 13:19. PMID: 29977330.
13. Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016; 1:196–206. PMID: 28404092.
14. Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006; 17:2974–2984. PMID: 17035611.
15. Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: evaluation of the critical issues. Liver Transpl. 2012; 18:1290–1301. PMID: 22847917.
16. Teperman LW, Poordad F, Bzowej N, Martin P, Pungpapong S, Schiano T, et al. Randomized trial of emtricitabine/tenofovir disoproxil fumarate after hepatitis B immunoglobulin withdrawal after liver transplantation. Liver Transpl. 2013; 19:594–601. PMID: 23447407.
17. Jiménez-Pérez M, Sáez-Gómez AB, Mongil Poce L, Lozano-Rey JM, de la Cruz-Lombardo J, Rodrigo-López JM. Efficacy and safety of entecavir and/or tenofovir for prophylaxis and treatment of hepatitis B recurrence post-liver transplant. Transplant Proc. 2010; 42:3167–3168. PMID: 20970638.
18. Park J, Jung KS, Lee HW, Kim BK, Kim SU, Kim DY, et al. Effects of entecavir and tenofovir on renal function in patients with hepatitis B virus-related compensated and decompensated cirrhosis. Gut Liver. 2017; 11:828–834. PMID: 28651305.
19. Riveiro-Barciela M, Tabernero D, Calleja JL, Lens S, Manzano ML, Rodríguez FG, et al. Effectiveness and safety of entecavir or tenofovir in a spanish cohort of chronic hepatitis B patients: validation of the page-B score to predict hepatocellular carcinoma. Dig Dis Sci. 2017; 62:784–793. PMID: 28078526.
20. Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: a systematic review and meta-analysis. Int Immunopharmacol. 2017; 42:168–175. PMID: 27915131.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download