Abstract
Purpose
Postoperative pancreatic fistula (POPF) is the most important factor affecting morbidity and mortality after pancreaticoduodenectomy (PD). Patients with a high controlling nutritional status (CONUT) score, which is used to assess nutritional status, are expected to have high morbidity rates. This study aimed to determine the usefulness of the CONUT score.
Methods
Data from 97 consecutive cases of PD performed in the Department of Surgery of Iwakuni Clinical Center, from April 2008 to May 2018, were included. Preoperative patient data, including sex, age, and hypertension, and postoperative complication data were collected to analyze pancreatic fistula occurrence.
Results
Of the 97 patients, 2 9 patients (29.8%) were diagnosed with POPF ≥ B, with 26 cases (26.8%) classified as grade B and 3 (3.1%) as grade C. The mortality rate was 2.1% (2 of 97). In the univariate analysis, a significant association was observed between POPF and the following factors: body mass index (BMI) ≥ 22 kg/m2, high CONUT score, nonpancreatic carcinoma, and CT attenuation values. In multivariate analysis, BMI ≥ 22 kg/m2 (odds ratio [OR], 6.16; P < 0.001), high CONUT score (OR, 3.77; P = 0.009), nonpancreatic carcinoma (OR, 5.72; P = 0.009), and CT attenuation values (late/early ratio) in the pancreas (OR, 9.07; P = 0.006) were independent risk factors for POPF.
Pancreaticoduodenectomy (PD) is the primary treatment for malignant tumors involving the pancreatic head, lower bile duct, and duodenal ampulla [1]. This procedure is technically difficult and highly invasive. Therefore, it is associated with a high morbidity and mortality rate [2]. The perioperative mortality rate still ranges from 0% to 5% [34]. The m ost important factor affecting morbidity and mortality after PD is the development of postoperative pancreatic fistula (POPF). In recent studies, the incidence of POPF is still high, which accounted for 11.4%–64.3% [56]. POPF is associated with delayed gastric emptying, intra-abdominal abscesses, surgical site infection, sepsis, and bleeding after PD [7]. Several approaches may reduce the incidence of pancreatic fistula (PF) [89]. However, to date, a definitive approach that prevents POPF has still not been established.
The controlling nutritional status (CONUT) score is an automatic tool to assess nutritional status, taking into account laboratory information, including serum albumin, total cholesterol level, and total lymphocyte count [10]. The CONUT score has been used to objectively evaluate nutritional status in patients with inflammatory disease, chronic heart failure, and chronic liver disease [1112]. Recently, the CONUT score was shown to be a predictive or prognostic marker in patients with malignancies, including colorectal, esophageal cancer, and hepatocellular carcinoma [131415]. However, the usefulness of the CONUT score in assessing the risk of POPF after PD has not been determined. Therefore, this retrospective study aimed to determine the significant preoperative predictive factors for POPF and to investigate whether the preoperative CONUT score could be a useful predictor of POPF.
Patients were not required to provide informed consent for the study as the analysis used anonymous data that were obtained after patients agreed to treatment by written consent. The ethics committee at the Iwakuni Clinical Center approved the study protocol (No. 0107). This study was performed in accordance with the protocols of the 1975 Declaration of Helsinki.
We reviewed the data from 97 consecutive PD cases performed in the Department of Surgery of Iwakuni Clinical Center from April 2008 to May 2018. The following preoperative patient data were collected: sex, age, hypertension, diabetes, alcohol consumption, smoking, body mass index, CONUT score, indication for surgery, main pancreatic duct (MPD) diameter by preoperative CT or magnetic resonance cholangiopancreatography, CT attenuation values in the pancreatic body, blood transfusion, blood loss, operative time, and pancreaticojejunostomy technique.
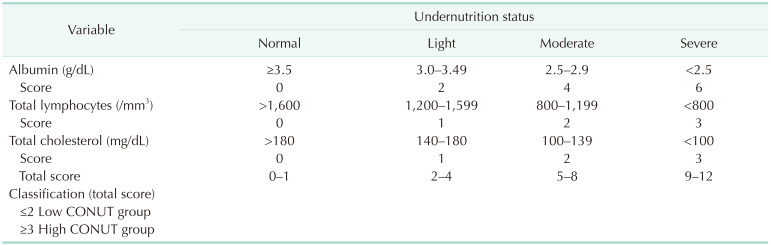
Preope rative blood samples were obtained within 2 weeks before surgery. The preoperative CONUT score was calculated using albumin level, total lymphocyte count, and total cholesterol level in each patient [10]. (1) A lbumin concentrations ≥3.5, 3.0–3.49, 2.5–2.99, and <2.5 g/dL were scored as 0, 2, 4, and 6 points, respectively. (2) Total lymphocyte counts ≥1,600, 1,200–1,599, 800–1,199, and <800/mm3 were scored as 0, 1, 2, and 3 points, respectively. (3) Total cholesterol concentrations ≥180, 140–179, 100–139, and <100 mg/dL were scored as 0, 1, 2, and 3 points, respectively. The CONUT score was defined as the sum of (1), (2), and (3) (Table 1). In this study, patients were divided into 2 groups: low CONUT group (score ≤ 2) and high CONUT group (score ≥ 3) [1314].
In addition, data on postoperative complication were collected for analysis with a focus on the occurrence of pancreatic fistula. No patient was excluded from the case series.
A soft pancreas is reported to be the risk factor for POPF [16]. However, pancreas firmness assessed by the surgeon alone during operation may not be accurate. It is obscure where the softness ends and firmness begins. Pancreatic fibrosis decreases the softness of the gland. Hashimoto reported that the ratio of the mean pancreatic CT attenuation value (hepatic to pancreatic phase; late/early [L/E ratio]) upstream from the tumor can assess the historical degree of pancreatic fibrosis [17]. Therefore, we used the CT attenuation value (L/E ratio) at the pancreatic body to assess pancreatic firmness.
Patients underwent subtotal stomach-preserving PD by 2 surgeons specializing in pancreatic surgery. All operations were performed via an open approach, and the degree of locoregional lymphadenectomy was determined according to the preoperative diagnosis. Surgical reconstruction was performed using a modification of the Child's method. The proximal jejunal stump was passed through the retrocolic pathway, and pancreaticojejunostomy, biliojejunostomy, and gastrojejunostomy were performed. Pancreaticojejunostomy was performed using the modified Kakita anastomosis (n = 47; April 2008–May 2013) or the modified Blumgart anastomosis (n = 50; June 2015–present) [23]. During the procedure, the surgeon decided whether plastic stents for internal drainage needed to be inserted into the MPD. Two or 3 abdominal drains were placed anterior or posterior to the pancreaticojejunostomy anastomosis and hepaticojejunostomy anastomosis.
POPF was diagnosed and graded in accordance with the International Study Group on Pancreatic Fistula classification [18]. POPF was diagnosed when the amylase concentration in the drainage fluid on postoperative day 3 was more than 3 times the upper limit of the normal serum level. POPF with an elevated inflammatory response noted in the blood examination and intravenous administration of antibiotics was defined as grade B POPF caused by infection. POPF that required drain placement for >22 days without an elevated inflammatory response or administration of antibiotics was defined as grade B POPF caused by long drain placement. Latent POPF [19] was defined as POPF that initially lacked amylase-rich effluent but ultimately progressed to clinically relevant POPF.
Statistical analyses were performed using the unpaired Student t-test and the chi-square test with Fisher exact test. All variables were assessed using univariate analyses, and only those variables showing statistical significance (P < 0.05) were evaluated using multivariate logistic analyses to determine the main independent risk factors for POPF. A value of P < 0.05 was considered statistically significant. Statistical analysis was undertaken using the JMP version 9 software (SAS Institute, Cary, NC, USA).
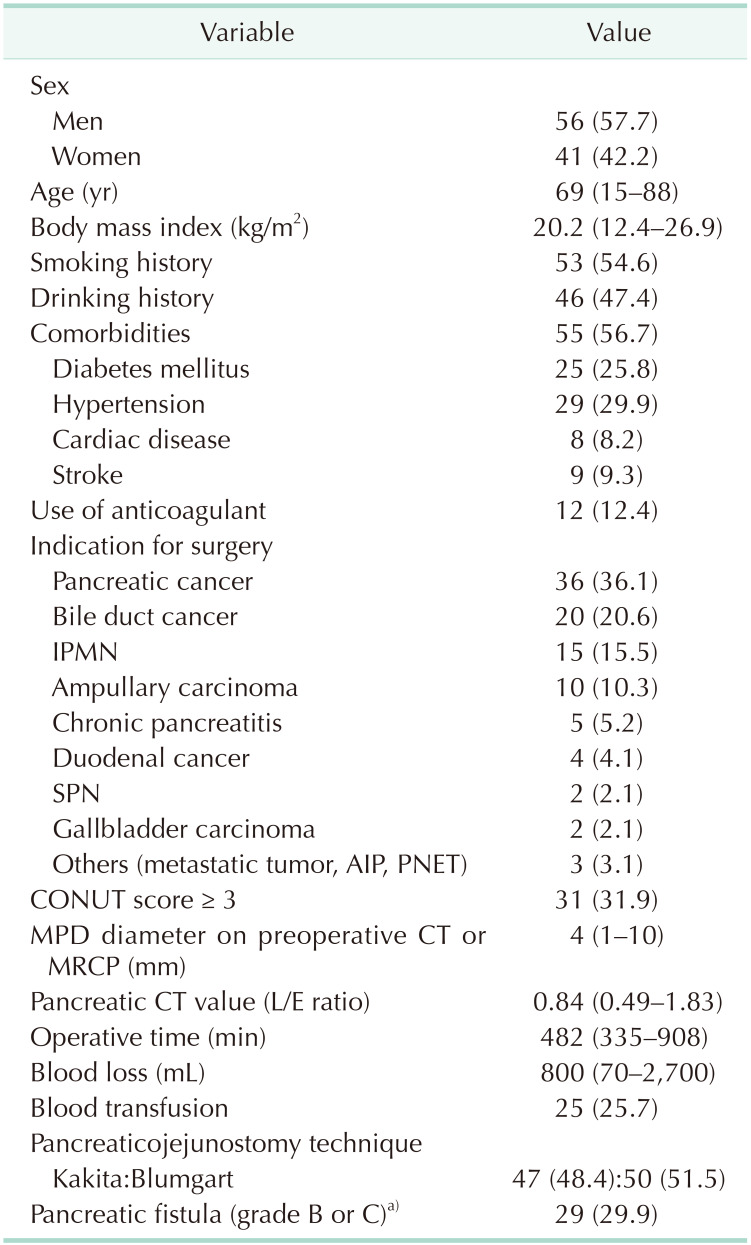
Clinical and preoperative characteristics of 97 patients are summarized in Table 2. The study population consisted of 56 men and 41 women, with a median age of 69 years (interquartile range, 15–88 years). The median CONUT score was 2 (interquartile range, 1–3) (Fig. 1). The low CONUT group included 61 patients (62.9%), and the high CONUT group included 36 patients (37.1%). The indication for PD included pancreatic carcinoma in 36 patients (37.1%), distal bile duct carcinoma in 20 (20.6%), intraductal papillary mucinous neoplasm in 15 (15.5%), ampullary carcinoma in 10 (10.3%), chronic pancreatitis in 5 (5.2%), duodenal carcinoma in 4 (4.1%), solid-pseudopapillary neoplasm in 2 (2.1%), gallbladder carcinoma in 2 (2.1%), and other diseases (metastatic tumor, pancreatic neuroendocrine tumor, autoimmune pancreatitis). The mean postoperative duration of hospital stay was 22 days (12–93 days). A total of 29 patients (29.8%) were diagnosed with POPF ≥ B, with 26 cases (26.8%) classified as grade B and 3 (3.1%) as grade C. There was no trend change of POPF according to the time period. The mortality rate among the study population was 2.1% (2 of 97). One died of aspiration pneumonia and sepsis associated with POPF, and another died of abdominal bleeding associated POPF.
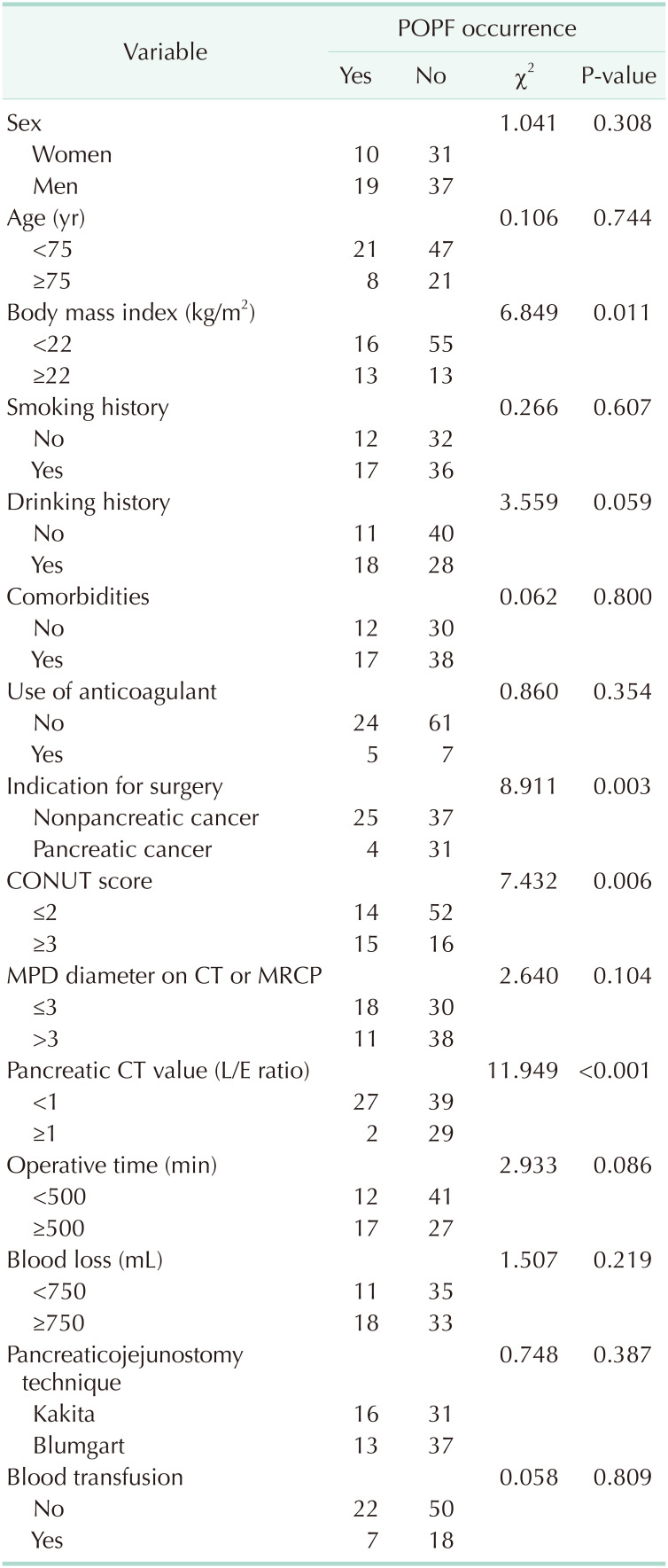
When the demographic and clinical variables were assessed using univariate analysis to determine the relationship with POPF, no statistical significance was found for age, sex, presence of hypertension, presence of diabetes mellitus, smoking history, and anticoagulant therapy (Table 3). A significant association was observed between POPF and the following factors: BMI ≥ 22 kg/m2 (P = 0.011), high CONUT score (P = 0.006), nonpancreatic carcinoma (P = 0.003), and CT attenuation values (L/E ratio) in the pancreatic body (P < 0.001).
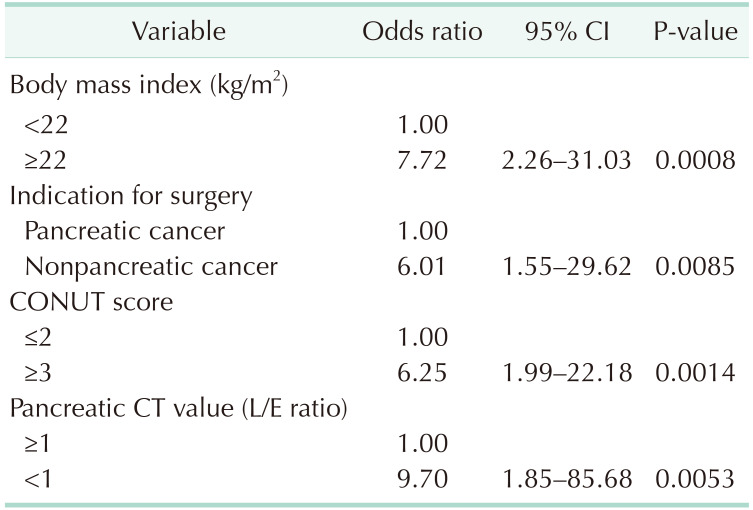
The preoperative risk factors for POPF (BMI ≥ 22 kg/m2, high CONUT score, nonpancreatic cancer, CT attenuation values [L/E ratio] in the pancreatic body) demonstrated in the univariate analysis were incorporated into the logistic regression analysis. The results showed that BMI ≥ 22 kg/m2 (odds ratio [OR], 7.72; P < 0.001), high CONUT score (OR, 6.25; P = 0.001), nonpancreatic carcinoma (OR, 6.01; P = 0.009 ), and CT attenuation values (L/E ratio) in the pancreatic body (OR, 9.70; P = 0.005) were independent risk factors for POPF (Table 4).
A simple scoring system for all patients was then developed, with 1 point assigned to each significant factor: BMI ≥ 22 kg/m2, high CONUT score, nonpancreatic carcinoma, and CT attenuation values (L/E ratio) in the pancreatic body, using similar OR to that used in the multivariate analysis. The patients were divided into 4 groups according to the number of risk factors. The proportion of POPF in each group was well categorized (Fig. 2).
In this study, the multivariate logistic regression analysis showed that high CONUT score, BMI ≥ 22 kg/m2, nonpancreatic carcinoma, and CT attenuation values (L/E ratio) in the pancreatic body were independent risk factors for POPF. In this study, we found a correlation between the CONUT score and POPF. Patients with a high CONUT score had significantly higher incidence of POPF. To our knowledge, this is the first study to investigate the relationship between CONUT score and incidence of POPF. The CONUT score is easily estimated using preoperative blood biochemical examination and is a useful tool in predicting the occurrence of POPF.
The CONUT score is an efficient tool for ear ly detection and continuous control of undernutrition in hospitalized patients, allowing nutritional status to be monitored in all inpatients [10]. This score is derived from 3 parameters; namely, serum concentrations of albumin (an indicator of protein reserve), total cholesterol (a caloric depletion parameter), and total lymphocyte count (an indicator of loss of immune defenses caused by malnutrition) [10]. Thus, the CONUT score presents 3 important immunonutritional indices.
POPF (grades B and C) is the most common and challenging PD complication, which has the potential to trigger a life-threatening delayed massive intra-abdominal hemorrhage and septicemia. Early prediction of this complication may improve postoperative monitoring of patients at a high risk of developing POPF. Predictors of POPF have been extensively studied. The following factors are reported to be related to POPF: sex, age, preoperative jaundice, intraoperative blood loss, low albumin level, high American Society of Anesthesiologists score, operative time, pancreatic texture, BMI, diameter of the MPD, and pancreaticojejunal anastomosis [620]. A multiinstitutional study confirmed the Fi stula Risk Score as a valid tool for predicting the development of POPF after PD. In particular, Callery et al. [21] reported that a simple 10-point Fistula Risk Score (based on small pancreatic duct, soft texture pancreas, high-risk pathology, and operative blood loss volume) is capable of precisely forecasting POPF after PD. Our scoring system can well categorize significant factors and be a useful tool for clinical practice. Those prediction strategies are easy, convenient, and amenable to widespread adoption so that surgeons may predict, diagnose, and treat POPF in a timely manner.
Compared with nonmalnourished patients, malnourished surgical patients have significantly higher incidence of postoperative complication such as SSI [22]. Therefore, besides the CONUT score, various screening tools have been developed to assess preoperative nutritional status [23]. Recently, Kim et al. [24] described that an integrated nutritional risk screening (NRS) system, including albumin, total lymphocyte count, BMI, and subjective risk factors (weight loss, dietary intake loss, and dysphagia), can be a preoperative predictive model for postoperative complication and found that the NRS risk group was independently associated with POPF. This suggests that a potential relationship exists between nutrition status and POPF.
Preoperative nutritional support such as immunonutrition decreased SSI incidence [25]. Therefore, in patients undergoing PD, interventions for nutritional status managem ent through preoperative immunonutrition may be necessary to decrease the incidence of POPF. Aida et al. [26] reported that preoperative immunonutrition modulates prostaglandin E2 production and T-cell differentiation and may protect against the aggravation of operative complication in patients undergoing PD. Recently, the enhanced recovery after surgery (ERAS) program including preoperative immunonutrition has been reported safe and contributed to decreased total complication rates in the hepatobiliary-pancreatic area and duration of hospital stay [27]. The underlying principle of the ERAS program is a multimodal perioperative protocol to attenuate the inflammatory response and potentiate patient rehabilitation after surgery [28]. To improve preoperative nutrition status, ad aptation of an ERAS program may be effective in patients with a high CONUT score to decrease the incidence of POPF.
An increased BMI has already been widely accepted as a patient-related risk factor that predisposes patients to POPF. The high incidence of POPF in patients with high BMI may lead to increased difficulty in exposing the pancreas during surgery due to a higher volume of abdominal fat and peripancreatic fat, a higher risk of damage to the pancreatic capsule during separation due to a soft and brittle pancreas, and a higher risk of pancreatic leakage caused by damage to the pancreatic tissue and fine pancreatic ducts due to suturing and knotting during pancreaticojejunal anastomosis [29].
In addition, a soft pancreas is a risk factor for POPF [16]. In this study, we assessed pancreatic firmness using the CT attenuation value (L/E ratio), which has been proposed to be associated with the texture of the pancreatic parenchyma. The L/E ratio positively correlated with pancreatic firmness, which reflects the histological degree of fibrosis in the pancreas. Our data showed that the L/E ratio was significantly lower in patients with POPF, and a soft pancreas was associated with POPF. The high incidence of POPF in patients with soft pancreas may lead to unsecure suturing and knotting, which can result in unsatisfactory pancreaticojejunal anastomosis, and a high risk of damage to the pancreatic tissue and fine pancreatic ducts during suturing and knotting of a soft pancreas, resulting in pancreatic leakage [29].
Nonpancreatic cancers such as ampullary carcinoma, bile duct carcinoma, or intraductal papillary mucinous neoplasm were indicated as risk factors, because these diseases clearly reflect the remnant pancreatic characteristics of the soft texture of the pancreas, a thin pancreatic body, and a nonfibrotic pancreatic parenchyma, which greatly increase the risk of POPF [30].
This study had some limitations. First, this was a retrospective, single-center study; there may be potential selection bias in the enrollment of patients for PD. Second, the sample size was small. Third, although the CONUT score conventionally described 4 classes of undernutrition, we used other cutoff values according to a previous report [1314]. In this study, the cutoff value for the CONUT score associated with POPF using receiver operating characteristics was also 3 (area under the curve = 0.61). Further studies are needed to determine more adequate cutoff values of the CONUT score to predict the incidence of POPF. Fourth, this study was based on a long-term chronologic experience. Finally, we did not compare the efficiency of the CONUT score with that of other screening systems. Further studies are required to assess the efficacy of screening systems to evaluate patients' status.
High CONUT score, BMI ≥ 22 kg/m2, nonpancreatic carcinoma, and CT attenuation values (L/E ratio) in the pancreatic body were found to be independent risk factors for POPF after PD. The CONUT score is an effective tool to assess preoperative nutritional status and predict the incidence of POPF after PD. The adaptation of the ERAS program including i mmunonutrition may be effective in patients with high CONUT scores to decrease the incidence of POPF.
ACKNOWLEDGEMENTS
This work was supported by the Department of Surgery at the Iwakuni Clinical Center.
References
1. Brown EG, Yang A, Canter RJ, Bold RJ. Outcomes of pancreaticoduodenectomy: where should we focus our efforts on improving outcomes? JAMA Surg. 2014; 149:694–699. PMID: 24849180.
2. Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 2014; 259:773–780. PMID: 24253151.
3. Fang Y, Gurusamy KS, Wang Q, Davidson BR, Lin H, Xie X, et al. Pre-operative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev. 2012; 9:CD005444.
4. Winter JM, Cameron JL, Yeo CJ, Alao B, Lillemoe KD, Campbell KA, et al. Biochemical markers predict morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2007; 204:1029–1036. PMID: 17481534.
5. Sugimoto M, Takahashi S, Gotohda N, Kato Y, Kinoshita T, Shibasaki H, et al. Schematic pancreatic configuration: a risk assessment for postoperative pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg. 2013; 17:1744–1751. PMID: 23975030.
6. Liu QY, Zhang WZ, Xia HT, Leng JJ, Wan T, Liang B, et al. Analysis of risk factors for postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastroenterol. 2014; 20:17491–17497. PMID: 25516663.
7. Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007; 11:1451–1458. PMID: 17710506.
8. Jang JY, Shin YC, Han Y, Park JS, Han HS, Hwang HK, et al. Effect of polyglycolic acid mesh for prevention of pancreatic fistula following distal pancreatectomy: a randomized clinical trial. JAMA Surg. 2017; 152:150–155. PMID: 27784046.
9. Kwon HJ, Ha HT, Choi YY, Kim SG. The effects of the end-to-side inverted mattress pancreaticojejunostomy on postoperative pancreatic fistula: a single surgeon's experience. Ann Surg Treat Res. 2015; 89:61–67. PMID: 26236694.
10. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005; 20:38–45.
11. Ueno T, Hirayama S, Ito M, Nishioka E, Fukushima Y, Satoh T, et al. Albumin concentrat ion determined by the modified bromocresol purple method is superior to that by the bromocresol green method for assessing nutritional status in malnourished patients with inflammation. Ann Clin Biochem. 2013; 50(Pt 6):576–584. PMID: 23897106.
12. Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, et al. Nutritional status is associated with inf lammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb. 2016; 23:713–727. PMID: 26782970.
13. Takagi K, Yagi T, Umeda Y, Shinoura S, Yoshida R, Nobuoka D, et al. Preoperative controlling nutritional status (CONUT) score for assessment of prognosis following hepatectomy for hepatocellular carcinoma. World J Surg. 2017; 41:2353–2360. PMID: 28389736.
14. Iseki Y, Shibutani M, Maeda K, Nagahara H, Ohtani H, Sugano K, et al. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PLoS One. 2015; 10:e0132488. PMID: 26147805.
15. Yoshida N, Harada K, Baba Y, Kosumi K, Iwatsuki M, Kinoshita K, et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbecks Arch Surg. 2017; 402:333–341. PMID: 28138759.
16. Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD. Risk Factors and Outcomes in Postpancreaticoduodenectomy Pancreaticocutaneous Fistula. J Gastrointest Surg. 2004; 8:951–959. PMID: 15585382.
17. Hashimoto Y, Sclabas GM, Takahashi N, Kirihara Y, Smyrk TC, Huebner M, et al. Dual-phase computed tomography for assessment of pancreatic fibrosis and anastomotic failure risk following pancreatoduodenectomy. J Gastrointest Surg. 2011; 15:2193–2204. PMID: 21948179.
18. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138:8–13. PMID: 16003309.
19. Pratt WB, Callery MP, Vollmer CM Jr. The latent presentation of pancreatic fistulas. Br J Surg. 2009; 96:641–649. PMID: 19434658.
20. Fu SJ, Shen SL, Li SQ, Hu WJ, Hua YP, Kuang M, et al. Risk factors and outcomes of postoperative pancreatic fistula after pancreatico-duodenectomy: an audit of 532 consecutive cases. BMC Surg. 2015; 15:34. PMID: 25887526.
21. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A Prospectively Validated Clinical Risk Score Accurately Predicts Pancreatic Fistula After Pancreatoduodenectomy. J Am Coll Surg. 2013; 216:1–14. PMID: 23122535.
22. Afaneh C, Gerszberg D, Slattery E, Seres DS, Chabot JA, Kluger MD. Pancreatic cancer surgery and nutrition management: a review of the current literature. Hepatobiliary Surg Nutr. 2015; 4:59–71. PMID: 25713805.
23. Arezzo di Trifiletti A, Misino P, Giannantoni P, Giannantoni B, Cascino A, Fazi L, et al. Comparison of the performance of four different tools in diagnosing disease-associated anorexia and their relationship with nutritional, functional and clinical outcome measures in hospitalized patients. Clin Nutr. 2013; 32:527–532. PMID: 23218121.
24. Kim JH, Lee H, Choi HH, Min SK, Lee HK. Nutritional risk factors are associated with postoperative complications after pancreaticoduodenectomy. Ann Surg Treat Res. 2019; 96:201–207. PMID: 30941324.
25. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Maeda S, Haraguchi N, et al. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann Surg Oncol. 2015; 22 Suppl 3:S778–S785. PMID: 26286199.
26. Aida T, Furukawa K, Suzuki D, Shimizu H, Yoshidome H, Ohtsuka M, et al. Preoperative Immunonutrition Decreases Postoperative Complications by Modulating Prostaglandin E2 Production and T-cell Differentiation in Patients Undergoing Pancreatoduodenectomy. Surgery. 2014; 155:124–133. PMID: 24589090.
27. Hughes MJ, McNally S, Wigmore SJ. Enhanced recovery following liver surgery: a systematic review and metaanalysis. HPB (Oxford). 2014; 16:699–706. PMID: 24661306.
28. Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008; 248:189–198. PMID: 18650627.
29. Hu BY, Wan T, Zhang WZ, Dong JH. Risk factors for postoperative pancreatic fistula: Analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. 2016; 22:7797–7805. PMID: 27678363.
30. Aoki S, Miyata H, Konno H, Gotoh M, Motoi F, Kumamaru H, et al. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: a nationwide study of 17,564 patients in Japan. J Hepatobiliary Pancreat Sci. 2017; 24:243–251. PMID: 28196308.
Fig. 2
Proportion of postoperative pancreatic fistula to the scoring system. A simple scoring system for all patients was then developed, with 1 point assigned to each significant factor: body mass index ≥ 22 kg/m2, high controlling nutritional status score, nonpancreatic carcinoma, and CT attenuation values (L/E ratio) in the pancreatic body, using similar odds ratio to that is used in the multivariate analysis. The patients were divided into 4 groups according to the number of risk factors. L/E ratio, late/early phase ratio.
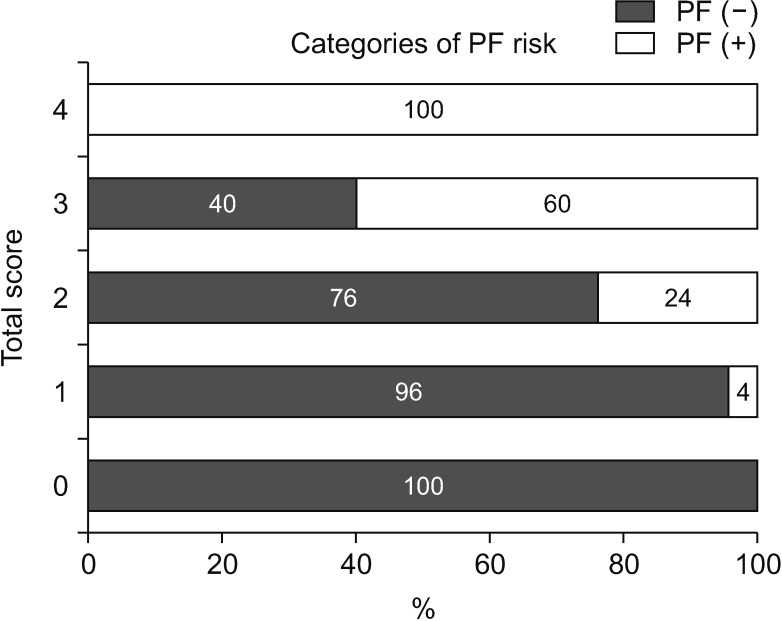
Table 2
Clinical and preoperative characteristics of 97 patients in this study
Values are presented as number (%) or median (interquartile range).
IPMN, intraductal papillary mucinous neoplasm; SPN, solid-pseudopapillary neoplasm; AIP, autoimmune pancreatitis; PNET, pancreatic neuroendocrine tumor; CONUT, controlling nutritional status; MPD, main pancreatic duct; MRCP, magnetic resonance cholangiopancreatography; L/E ratio, late/early phase ratio.
a)According to the international Study Group of Pancreatic Fistula (ISGPF) classification.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download