Abstract
Purpose
A variety of clinical features of anastomotic leak occur during the surgical treatment of rectal cancer. However, little information regarding management of leakage is available and treatment guidelines have not been validated. The aim of this study was to evaluate the validity of currently proposed expert opinions on the management of anastomotic leak, after low anterior resection for rectal cancer.
Methods
A retrospective analysis was conducted for 1,786 patients who underwent sphincter-preserving surgery for rectal cancer between 2005 and 2015. Clinical outcomes including anastomotic leak-associated mortality and permanent stoma were analyzed.
Results
The overall incidence of anastomotic leak was 6.8% (122 of 1,786), including 6.1% (30 of 493 patients) with diverting stoma and 7.1% (92 of 1,293 patients) without diverting stoma (P = 0.505). A majority of patients without diversion were treated with diverting stoma (76 of 88 patients [86.4%]); 1 mortality (0.8%) was observed in this group. Treatments in the diversion group mainly included conservative treatment, local drainage, and/or transanal repair (26 of 30 patients [86.7%]). The anastomotic failure rates were 20.7% (19 of 92 patients) in the no diversion group and 53.3% (16 of 30 patients) in the diversion group. In the multivariate analysis, preoperative chemoradiotherapy (P < 0.001) and delayed diagnosis of anastomotic leak (P = 0.036) were independent risk factors for permanent stoma.
Go to : 
Anastomotic leak (AL) is the most feared complication after rectal surgery and can cause significant morbidity and mortality [12]. Despite the controversial results among different studies, some authors hold the view that AL is associated with increased local recurrence and reduction in patient survival in the treatment of rectal cancer [34]. Furthermore, AL can subsequently result in prolonged anastomotic sequelae such as fistula, sinus, and stricture, which markedly impair a patient's quality of life and lead to the need for a permanent stoma [56].
Due to the wide variety of clinical features of AL encountered in the modern treatment of rectal cancer, surgeons must know the outcome of their chosen treatment option. Since the initial treatment selected for the management of AL has been shown to influence the patient outcome, factors such as the presence or absence of generalized peritonitis, presence or absence of a diverting stoma, patient characteristics (sex, age, and comorbidities), tumor stage, anastomotic height, anastomotic defect size, use of chemoradiotherapy (CRT), and type of surgery (laparoscopic vs. open method) can all be considered during treatment. In this regard, 2 guidelines using the Delphi methodology that provide expert opinions on this issue have been published; one from the International Anastomotic Leak Study Group [7] and the other from the Association of Surgeons of Great Britain and Ireland [8]. The American Society of Colon and Rectal Surgeons has also published a similar guideline [9]. However, little information on the outcomes of procedures adherent to these guidelines is available. Therefore, the aim of this study was to evaluate the validity of the current proposed expert opinions. Further, we also investigated the incidence and risk factors of AL-associated requirement for a permanent stoma, with a specific focus on clinical factors and treatment selection.
Go to : 
This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (No. cnuhh-2020-088). All patients gave their informed consent in writing prior to surgery during the study period. Between January 2005 and June 2015, the medical data of 1,786 patients who underwent sphincter-preserving surgery for rectal cancer at Chonnam National University Hwasun Hospital were collected and reviewed. All patients had a pathologically confirmed rectal adenocarcinoma located within 15 cm from the anal verge. The tumor height was evaluated using rigid sigmoidoscopy. Mechanical bowel preparation was performed in all patients. The surgical technique has been described previously [10]. Preoperative and postoperative CRT were also applied according to previously described criteria [11]. CRT was administered at 4,500–5,040 cGy in 25–28 fractions delivered to the tumor-bearing area.
AL was defined as a disruption of the anastomosis line identified on reoperation, rigid sigmoidoscopy, and CT. The presence of AL was clinically suspected and investigated when the patient had abdominal pain, tenderness, rebound tenderness, fever, pus or fecal discharge from the pelvic drain, leukocytosis, and elevated CRP. Clinical variables associated with AL were investigated, including the severity of peritoneal contamination during laparoscopy or laparotomy, extent of anastomotic defect as assessed using rigid sigmoidoscopy, time to the diagnosis of AL, and type of treatment for AL. The severity of peritoneal contamination was categorized as described by Damrauer et al. [12], at reoperation or by reviewing the CT scans; contained leaks were defined as purulent discharge confined in the pelvis or perianastomotic space, and diffuse leaks as those when diffuse gross contamination was found in the peritoneal cavity. The cutoff for defining an anastomotic defect was a defect of more than one-third of the circumference of the anastomosis or a defect size of >2 cm.
The treatment strategy for AL was dependent on the presence of a previous diverting stoma. In patients without a previous diverting stoma, anastomotic resection and redo anastomosis were performed in the presence of large anastomotic defect, extensive peritoneal contamination, or overt neorectal ischemia, with formation of a diverting stoma. If the patient was hemodynamically unstable, a Hartmann operation was performed. If the defect was small or only clinically suspected and not discovered intraoperatively or endoscopically, a diverting stoma was created and primary suture of the anastomotic defect was carried out transanally or laparoscopically depending on the height of the anastomosis. In patients who had a diverting stoma, we preferred performing transanal drainage. Stoma takedown was attempted after checking the healing of the anastomotic defect through water-soluble contrast enema and rigid sigmoidoscopy. When AL was suspected by extraluminal spread of water-soluble contrast enema without overt symptoms (n = 2), stoma takedown was postponed. However, stoma takedown was attempted in patients (n = 1) showing an asymptomatic chronic sinus after deroofing the sinus.
The chi-squared test or Fisher exact test was used to analyze the significance of categorical variables, and Student t-test was used to analyze continuous variables. Univariate logistic regression analysis was used to identify the predictors of a permanent stoma. Variables that were significant at P < 0.10 in the univariate analysis were considered in a backward stepwise multivariate logistic regression model with calculation of the odds ratios (ORs) and 95% confidence intervals (CIs). A P-value of <0.05 was considered significant. Statistical analysis was performed using R statistical software, ver. 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org).
Go to : 
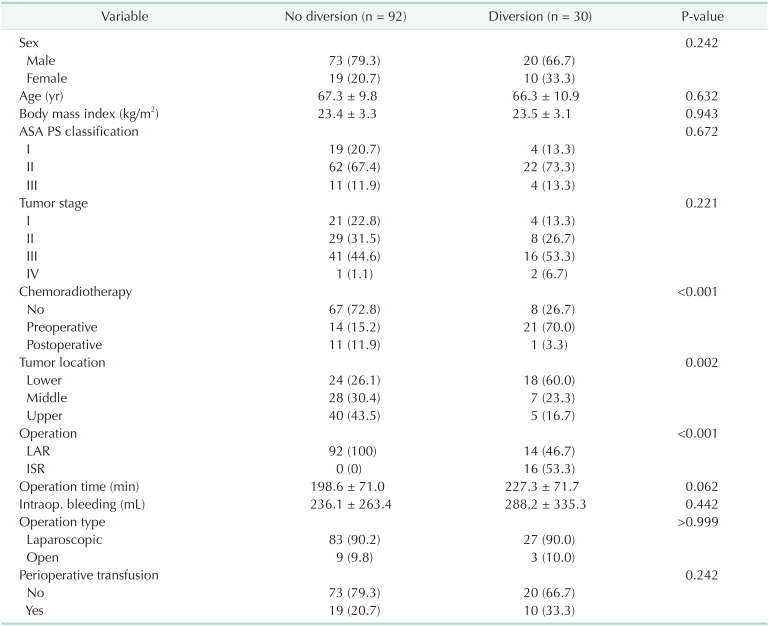
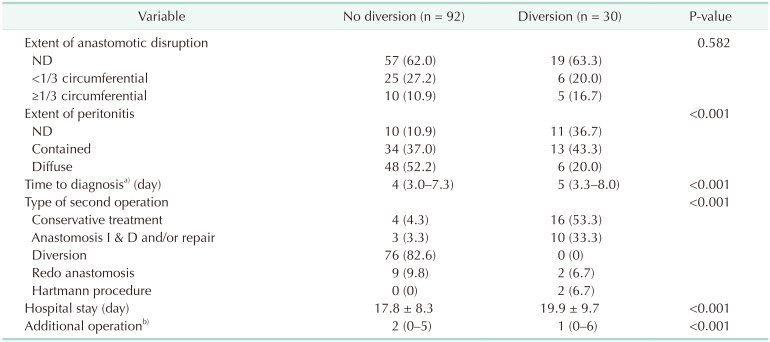
During the study period, 1,786 patients underwent sphincter-preserving surgery for rectal cancer. The overall incidence of AL was 6.8% (122 of 1,786 patients), including 6.1% (30 of 493 patients) in the group with a diverting stoma (diversion group) and 7.1% (92 of 1,293 patients) in the group without a diverting stoma (no diversion group). The median period from initial surgery to diagnosis of AL was 5 days (interquartile range, 3–16 days). Twelve patients took postoperative CRT and all AL was diagnosed before the initiation of postoperative CRT (range, 3–19 days after surgery). Table 1 summarizes the clinical characteristics of patients who experienced AL, according to the presence of a diverting stoma created at initial surgery. The diversion group showed a higher proportion of preoperative CRT (P = 0.001), lower tumor location (P = 0.002), and more frequent use of intersphincteric resection (P < 0.001) than the no diversion group. In addition, the clinical parameters after the development of AL were also evaluated and compared (Table 2). The diversion group showed less severe peritoneal contamination, delayed diagnosis of AL, longer hospital stay, and more additional surgeries for AL treatment. However, the size of the anastomotic defect evaluated using sigmoidoscopy was not different between the 2 groups.
The treatment strategy for AL was determined by the presence or absence of a diverting stoma (Fig. 1). Most patients had no previous diversion and were mainly treated with formation of a diverting stoma. In the group without a previous diverting stoma, 86.4% (76 of 88 patients) underwent diversion surgery after the diagnosis of AL; however, stoma reversal was not pursued in 4 patients and re-stoma creation after a successful stoma takedown was inevitable in 10 patients. In addition, 3 of 7 patients treated with antibiotics and perianal drainage and 2 of 9 patients treated with redo anastomosis and diversion eventually needed a permanent stoma. As a result, the anastomotic failure rate was 20.7% (19 of 92 patients) in the no diversion group. On the other hand, the treatment for AL in the diversion group was mainly conservative treatment or local drainage and/or transanal repair. Excluding 2 patients treated with redo anastomosis and another 2 patients treated with Hartmann operation, a total of 86.7% (26 of 30 patients) were treated with this strategy. After a median interval of 6.7 months, stoma reversal was possible in patients (8 of 26) who had shown spontaneous healing of the anastomotic defect, excluding 1 patient who refused reversal. Persistent anastomotic complications were observed in 60.0% (18 of 30) of patients. Although stoma reversal was pursued in 10 patients, re-stoma creation was needed in 4 patients. As a result, 53.3% (16 of 30) of the patients in this group could not avoid a permanent stoma.
Redo anastomosis was adopted as initial treatment for AL in 9 (9.8%) in patients without diversion and 2 (6.7%) in diversion group (P = 0.661). The length of hospital stay was significantly longer in patients treated with this strategy than in patients who received any other treatment (median 25 days vs. 16 days, P = 0.035).
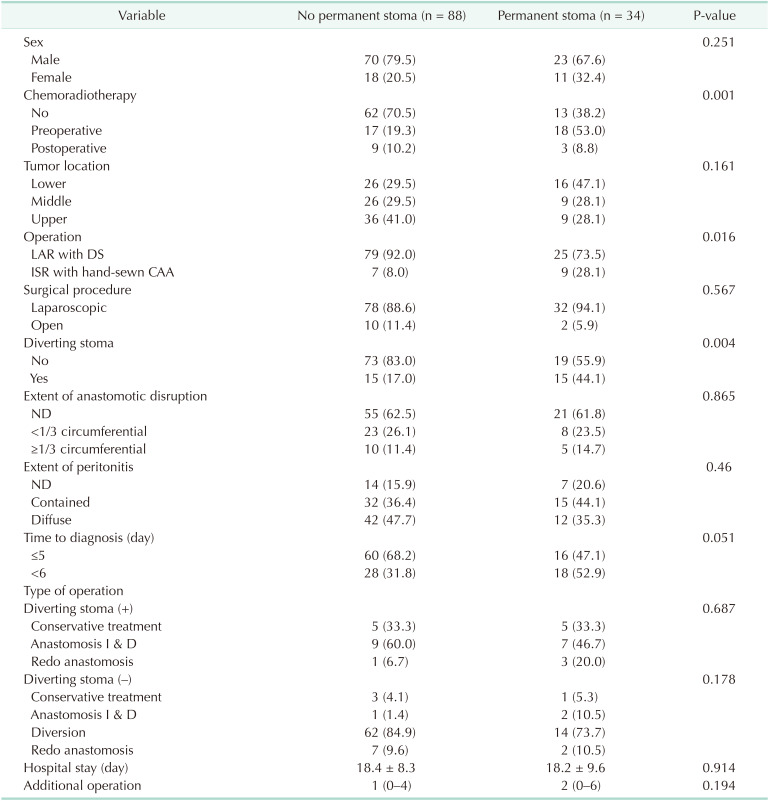
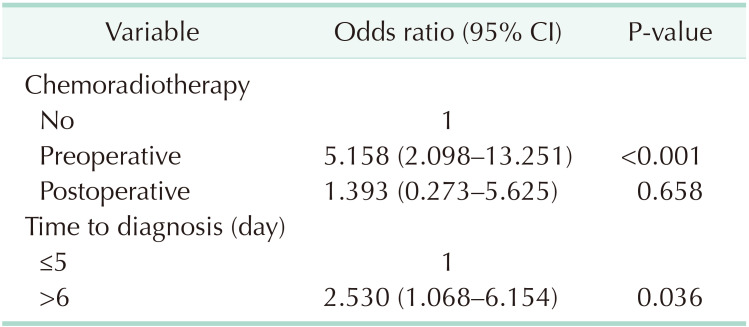
The clinical characteristics of the patients with AL according to the final presence of a stoma are summarized in Table 3. Permanent stoma was associated with preoperative CRT (P = 0.001), intersphincteric resection with hand-sewn anastomosis (P = 0.016), presence of diversion (P = 0.004), and delayed diagnosis of AL (P = 0.051). The extent of anastomosis disruption and the type of surgical treatment in each group were not associated with this clinical outcome. In multivariate analysis, preoperative CRT (OR, 5.158; 95% CI, 2.098–13.251; P < 0.001) and delayed diagnosis of AL (OR, 2.530; 95% CI, 1.068–6.154; P = 0.036) were independent risk factors of permanent stoma (Table 4).
Go to : 
In the present study, the overall incidence of AL was 6.8% and there was 1 reported mortality (0.8%). About 21% (19 of 92) of patients in the no diversion group and 53.3% (16 of 30) of patients in the diversion group could not avoid having a permanent stoma. The need for a permanent stoma associated with anastomotic complications after AL was predicted on the basis of the presence of the following 2 clinical factors; preoperative CRT and delayed onset of AL.
In an acute clinical setting, it is imperative to avoid or control sepsis, which is the major cause of morbidity and mortality in the management of AL. In this study, there was 1 case (0.8%) of death after AL, which is consistent with previous reports in which the rate of death after AL ranged from 0.4% to 12% [2131415]. The low death rate after AL in this study provides evidence for the efficacy of the initial source control and rescue treatment proposed in the surgical literature [7813]. Dismantlement of the anastomosis and formation of an end colostomy may be the most thorough surgical management for avoiding mortality, and should be considered as the first treatment option for patients with severe sepsis. However, many surgeons hold the opinion that restoration of bowel continuity after Hartmann operation is technically difficult and risky, especially in patients who underwent ultralow anterior resection or intersphincteric resection, which often results in the need for a permanent stoma [2131617]. Furthermore, patients may be hesitant to undergo reversal surgery after experiencing a long recovery time in the intensive care unit and a long rehabilitation period [2]. Boyce et al. [13] reported that 4 of 44 patients (9.1%) with AL after laparoscopic anterior resection were treated using Hartmann operation. Blumetti et al. [17] also reported that all 3 of their patients treated with Hartmann operation could not avoid a permanent stoma. In this regard, we attempted to perform Hartmann operation as selectively as possible, without increasing the mortality rate; 2 of 89 (2.2%) patients underwent urgent Hartmann operation and another 2 patients who had undergone redo anastomosis were eventually treated by this surgery for chronic anastomotic complications. In fact, all 4 patients who underwent Hartmann operation eventually had a permanent colostomy.
In the absence of severe septic shock, another important issue is to preserve bowel continuity. Some experts suggest that if the neorectum is not ischemic (i.e., viable), fecal diversion and pelvic drainage can successfully manage AL without further manipulation of the anastomosis or with minimal procedures (suture and/or drainage), and with a favorable spontaneous healing rate ranging from 54% to 100% [21718]. Above all, this strategy can be done simply and quickly. Several factors, however, should be considered to preserve bowel continuity in the treatment of AL. The selection can be based on the size of the defect. The American Society of Colon and Rectal Surgeons suggests that AL can be managed with fecal diversion if the size of the anastomotic defect is less than one-third of the circumference [9]. The size of the AL can be categorized according to the defect size (i.e., >1 cm, 2 cm) or the circumference relative to the anastomosis (i.e., more than one-third or one-half) [7]. Adapting this strategy (in which major defect is defined as one-half of the whole circumference), Parc et al. [2] reported that bowel continuity was restored in all 9 patients who had a defunctioning stoma, but in only 11 (57.9%) patients who had a Hartmann operation. Especially, this more conservative management was mainly feasible in patients with a low anastomosis (below the Douglas pouch) [2]. Recent studies adapting the laparoscopic technique and preoperative CRT in the treatment of rectal cancer have proposed this treatment [13171819]. Boyce et al. [13] reported that 21 of 24 (87.5%) patients who needed relaparotomy were treated with this approach, and 18 (85.7%) patients could avoid a permanent stoma. In this study, 76 patients who had no diverting stoma in the index surgery were initially treated with diversion and drainage. All these patients had an anastomotic defect <2 cm or one-third of the whole circumference. Almost all of these patients were candidates for stoma reversal, and bowel continuity was successfully achieved in 77.8% without further surgery. If the redo surgery (n = 3), strictuloplasty (n = 2), and re-stoma and reversal (n = 1) cases were included, >85% of patients could avoid a permanent stoma. On the basis of our results, we suggest that the size of the AL can be used as a useful indicator of whether or not the anastomosis should be broken down.
When there is evidence of ischemia at the anastomosis site and large or complete avulsion of the anastomosis, new (redo) colorectal or coloanal anastomosis can be considered as the last option to avoid a permanent stoma. Previous studies reported that the success rate of redo anastomosis ranged from 66% to 100% [20212223]. However, surgeons should keep in mind that it can cause further AL with an even higher risk than that at the time of the index surgery. In a study confined to patients who experienced AL, redo anastomosis not only had the lowest success rate (66%) but was also associated with a second AL rate of up to 41% [20]. In this study, a total of 11 (9 in the no diversion group, 2 in the diversion group) redo anastomoses were performed for the acute management of AL, and 8 patients (72.7%) could avoid a permanent stoma. As described by Westerduin et al. [20], the success rate will be worse in patients with preoperative CRT and in the management for acute AL than when it is performed for the management of chronic complications or in nonirradiated tissue.
Nonoperative management of acute AL was possible in the group with a diverting stoma, which more likely comprised patients with low-lying (extraperitoneal) anastomosis. Our results support this strategy, as the majority of patients who had a diverting stoma (86.7%) were successfully managed without re-laparotomy. The effectiveness of this management, which consisted of antibiotic treatment alone or transanal drainage, has been separately confirmed in previous studies [172425].
An unexpected finding was that in patients with a diverting stoma, the permanent stoma rate was high. In the current study, the rate of permanent stoma in the diversion group was 53.3% (16 of 30), whereas it was 13.9% in patients in the no diversion group whose AL was managed with fecal diversion and drainage. As stated above, Matthiessen et al. [25] reported that 5 of 12 (41.7%) patients could not avoid a permanent stoma. Other studies also reported a permanent stoma rate of >25% in this patient group [1324]. We can suggest possible explanations for the very high rate of permanent stoma in patients with a diverting stoma. On the basis of our multivariate analysis, preoperative CRT and delayed diagnosis of AL (resulting in treatment delay) were independent risk factors of a permanent stoma. Our data showed that the diversion group included many more patients who were treated with preoperative CRT and that the time of diagnosis in this group was statistically later than that in the no diversion group. Although whether preoperative CRT is a risk factor for AL after rectal cancer surgery is still controversial [2627], we believe that the deleterious effects of preoperative CRT become more dominant in inflamed tissue than in noninflamed tissue, especially when devitalized tissue and abscess are not promptly removed. Another plausible explanation is that radiation can cause a reduction of blood flow, which then results in a reduction of cytokines necessary for the recruitment of fibroblasts for the wound-healing process [28]. In addition, late radiation-related toxicity to the rectal mucosa can be another reason for the need for a permanent stoma. This usually occurs from 3 months to sometimes several years after radiotherapy [29]. We believe that late radiation-related toxicity could be associated with the need for re-stoma creation after successful repair of the diverting stoma, as well as with chronic anastomotic complications such as stricture, fistula, and sinus, which often result in a permanent stoma [6]. Consequently, although the defect size was not different according to the presence or absence of a diverting stoma, and even if the peritonitis was less severe, spontaneous healing of AL was less commonly achieved in this patient group. Finally, late-onset AL was more frequent in patients with a diverting stoma and was associated with a higher permanent stoma rate. This suggests that AL in this group might have shown less prominent symptoms and signs, whereas AL in the group without a diverting stoma caused more prominent symptoms and signs and needed prompt treatment or use of an additional diagnostic tool.
This study has some limitations. First, the observational nature of this study makes it susceptible to bias and unknown confounding. To reduce confounding, we performed multivariate analysis. Second, the size of the defect was measured by each surgeon without an objective measurement instrument and showed a tendency to gradually increase on repeated sigmoidoscopic examinations. Third, although we recorded the cases of patients who needed a stoma owing to compromised anal function, we observed a wide variability in the degree of anal sphincter function among patients. In this regard, the true impact of AL on quality of life and anal function was not tested in this study. Also, new promising treatments (i.e., endo-sponge treatment) were not included in this study. Despite these limitations, the present study can provide valuable clues for further studies on this topic.
In conclusion, with advancing knowledge and surgical techniques such as total mesorectal excision, preoperative CRT, and subcentimeter distal margin, the proportion of patients undergoing rectal cancer surgery with sphincter preservation and very low rectal anastomosis is increasing. Accordingly, the more diverse clinical presentations of AL require surgeons to tailor the management of this condition according to individual patient characteristics. Here, we showed and validated the results of AL management based on current expert opinions, and observed very low mortality and morbidity rates. The analysis of risk factors identified in this study for the need for a permanent stoma after the occurrence of AL will provide useful information for perioperative management and decision making personalized to each patient.
Go to : 
References
1. Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998; 85:355–358. PMID: 9529492.
2. Parc Y, Frileux P, Schmitt G, Dehni N, Ollivier JM, Parc R. Management of postoperative peritonitis after anterior resection: experience from a referral intensive care unit. Dis Colon Rectum. 2000; 43:579–589. PMID: 10826415.
3. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011; 253:890–899. PMID: 21394013.
4. Ptok H, Marusch F, Meyer F, Schubert D, Gastinger I, Lippert H, et al. Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg. 2007; 94:1548–1554. PMID: 17668888.
5. Bittorf B, Stadelmaier U, Merkel S, Hohenberger W, Matzel KE. Does anastomotic leakage affect functional outcome after rectal resection for cancer? Langenbecks Arch Surg. 2003; 387:406–410. PMID: 12607120.
6. Lee SY, Kim CH, Kim YJ, Kim HR. Anastomotic stricture after ultralow anterior resection or intersphincteric resection for very low-lying rectal cancer. Surg Endosc. 2018; 32:660–666. PMID: 28726144.
7. Phitayakorn R, Delaney CP, Reynolds HL, Champagne BJ, Heriot AG, Neary P, et al. Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J Surg. 2008; 32:1147–1156. PMID: 18283511.
8. McDermott FD, Arora S, Smith J, Steele RJC, Carlson GL, Winter DC. Issues in professional practice: prevention, diagnosis and management of colorectal anastomotic leakage [Internet]. London: Association of Coloproctology of Great Britain and Ireland;2016. cited 2020 Jul 30. Available from: http://www.acpgbi.org.uk/content/uploads/2016/03/management-of-colorectal-anastomtic-leakage.pdf.
9. Beck DE, Wexler SD, Hull TL, Roberts PL, Saclarides TJ. The ASCRS manual of colon and rectal surgery. 2nd ed. New York: Springer;2014.
10. Kim CH, Kim HJ, Huh JW, Kim YJ, Kim HR. Learning curve of laparoscopic low anterior resection in terms of local recurrence. J Surg Oncol. 2014; 110:989–996. PMID: 25292364.
11. Kim CH, Lee SY, Kim HR, Kim YJ. Pathologic stage following preoperative chemoradiotherapy underestimates the risk of developing distant metastasis in rectal cancer: a comparison to staging without preoperative chemoradiotherapy. J Surg Oncol. 2016; 113:692–699. PMID: 26914147.
12. Damrauer SM, Bordeianou L, Berger D. Contained anastomotic leaks after colorectal surgery: are we too slow to act? Arch Surg. 2009; 144:333–338. PMID: 19380646.
13. Boyce SA, Harris C, Stevenson A, Lumley J, Clark D. Management of low colorectal anastomotic leakage in the laparoscopic era: more than a decade of experience. Dis Colon Rectum. 2017; 60:807–814. PMID: 28682966.
14. Tevis SE, Carchman EH, Foley EF, Heise CP, Harms BA, Kennedy GD. Does anastomotic leak contribute to high failure-to-rescue rates? Ann Surg. 2016; 263:1148–1151. PMID: 26587851.
15. Shiomi A, Ito M, Maeda K, Kinugasa Y, Ota M, Yamaue H, et al. Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1,014 consecutive patients. J Am Coll Surg. 2015; 220:186–194. PMID: 25529899.
16. Lindgren R, Hallböök O, Rutegård J, Sjödahl R, Matthiessen P. What is the risk for a permanent stoma after low anterior resection of the rectum for cancer?: a sixyear follow-up of a multicenter trial. Dis Colon Rectum. 2011; 54:41–47. PMID: 21160312.
17. Blumetti J, Chaudhry V, Cintron JR, Park JJ, Marecik S, Harrison JL, et al. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg. 2014; 38:985–991. PMID: 24305917.
18. Hedrick TL, Sawyer RG, Foley EF, Friel CM. Anastomotic leak and the loop ileostomy: friend or foe? Dis Colon Rectum. 2006; 49:1167–1176. PMID: 16826334.
19. Joh YG, Kim SH, Hahn KY, Stulberg J, Chung CS, Lee DK. Anastomotic leakage after laparoscopic protectomy can be managed by a minimally invasive approach. Dis Colon Rectum. 2009; 52:91–96. PMID: 19273962.
20. Westerduin E, Borstlap WA, Musters GD, Westerterp M, van Geloven AAW, Tanis PJ, et al. Redo coloanal anastomosis for anastomotic leakage after low anterior resection for rectal cancer: an analysis of 59 cases. Colorectal Dis. 2018; 20:35–43. PMID: 28795776.
21. Genser L, Manceau G, Karoui M, Breton S, Brevart C, Rousseau G, et al. Postoperative and long-term outcomes after redo surgery for failed colorectal or coloanal anastomosis: retrospective analysis of 50 patients and review of the literature. Dis Colon Rectum. 2013; 56:747–755. PMID: 23652749.
22. Lefevre JH, Bretagnol F, Maggiori L, Ferron M, Alves A, Panis Y. Redo surgery for failed colorectal or coloanal anastomosis: a valuable surgical challenge. Surgery. 2011; 149:65–71. PMID: 20451231.
23. Pitel S, Lefèvre JH, Tiret E, Chafai N, Parc Y. Redo coloanal anastomosis: a retrospective study of 66 patients. Ann Surg. 2012; 256:806–811. PMID: 23095625.
24. Sirois-Giguère E, Boulanger-Gobeil C, Bouchard A, Gagné JP, Grégoire RC, Thibault C, et al. Transanal drainage to treat anastomotic leaks after low anterior resection for rectal cancer: a valuable option. Dis Colon Rectum. 2013; 56:586–592. PMID: 23575397.
25. Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007; 246:207–214. PMID: 17667498.
26. Qin C, Ren X, Xu K, Chen Z, He Y, Song X. Does preoperative radio(chemo) therapy increase anastomotic leakage in rectal cancer surgery?: a meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2014; 2014:910956. PMID: 25477955.
27. Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, et al. Impact of preoperative radiotherapy on anastomotic leakage and stenosis after rectal cancer resection: post hoc analysis of a randomized controlled trial. Dis Colon Rectum. 2016; 59:934–942. PMID: 27602924.
28. Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol. 1997; 42:99–106. PMID: 9106919.
29. Denham JW, Hauer-Jensen M. The radiotherapeutic injury: a complex ‘wound’. Radiother Oncol. 2002; 63:129–145. PMID: 12063002.
Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download