Abstract
Purpose
Previous studies have reported that progressive muscle loss, known as sarcopenia, has a negative impact on colon cancer treatment. However, the majority of studies have analyzed on patients undergoing open resection, and the association of sarcopenia with clinical outcomes is not clear for patients with colon cancer undergoing laparoscopic surgery. Thus, the aim of this study was to evaluate the impact of sarcopenia on clinical outcomes after laparoscopic surgery for colon cancer.
Methods
A total of 423 patients who underwent laparoscopic surgery for colon cancer between November 2010 and October 2014 were included. Body composition was assessed by measuring muscle and fat areas at the third lumbar vertebra (L3) on preoperative computed tomography. The L3 skeletal muscle area was used to calculate the skeletal muscle index and to assess for sarcopenia.
Results
Sarcopenia was identified in 54 patients (12.8%). The median time to first flatus (3 days), median time to tolerable soft diet (4 days), and median length of hospital stay (7 days) were not significantly different between patients with and without sarcopenia. However, sarcopenia was an independent risk factor for postoperative complications in the logistic regression multivariate analysis (P = 0.015). Sarcopenia was not associated with overall or disease-free survival.
Go to : 
Frailty is defined as a biological syndrome characterized by decreased reserve and resilience to stress factors across multiple physiological systems, and has been shown to be associated with adverse health outcomes [1]. A hallmark of frailty is sarcopenia, defined as the involuntary, progressive, and generalized loss of skeletal muscle mass with aging [2]. Sarcopenia has been known to have a negative impact on surgical complications and survival [34]. These negative impacts have also been noted in patients with colorectal cancer [567].
In colorectal cancer surgery, abdominal CT is routinely performed for staging work-up. In addition, abdominal CT imaging enables evaluation of patients' body composition and allows for identification of sarcopenia [78]. Sarcopenia was defined by using sex-specific cut-off points for the third lumbar vertebra (L3) skeletal muscle index (SMI). L3 level is used for evaluating sarcopenia due to its accuracy reflecting the real muscle mass and fat volume [3]. So far, previous studies regarding colorectal resection have demonstrated that sarcopenia has a negative impact on postoperative morbidity and survival [56789]. However, these studies included mostly patients undergoing open resection and those with metastases.
Recently, laparoscopic surgery has been used worldwide since the oncologic stability and clinical benefits were reported by several international, multicenter, randomized, and controlled trials [101112]. However, few studies have investigated the association of body composition with clinical outcomes after laparoscopic colorectal surgery. One previous study showed that laparoscopic surgery could reduce the negative impact of sarcopenia on short-term outcomes in patients with colorectal cancer, contrary to traditional open surgery [13]. However, the study only evaluated perioperative outcomes and did not assess oncologic results.
The aim of this study was to analyze the preoperative CT images of patients who underwent laparoscopic surgery for colon cancer, and to analyze the impact of sarcopenia on short-term and oncologic outcomes after laparoscopic surgery.
Go to : 
This retrospective study was approved by the Institutional Review Board (IRB) of Chungnam National University Hospital (NO. 2018-05-002) and the informed consent was waived by IRB. The medical records and preoperative CT images of patients who underwent laparoscopic surgery for colon cancer with curative intent between November 2010 and October 2014 at our institution were reviewed retrospectively. Exclusion criteria were as follows: (1) distant metastases at initial diagnosis, (2) previous radiotherapy or chemotherapy, (3) no available CT examination at initial diagnosis, and (4) conversion to open surgery. Nine patients were converted to open surgery because of duodenal invasion during right hemicolectomy (n = 2), huge tumor size (n = 3), and uncontrolled bleeding (n = 4).
Preoperative work-up included a complete history taking, a physical examination, complete blood count, liver function tests, colonoscopy, and CT scanning. Curative laparoscopic surgery was performed after mechanical bowel preparation and followed the principle of primary tumor excision. The completeness of resection was defined based on the operative and pathologic reports. All operative specimens had negative gross and microscopic margins.
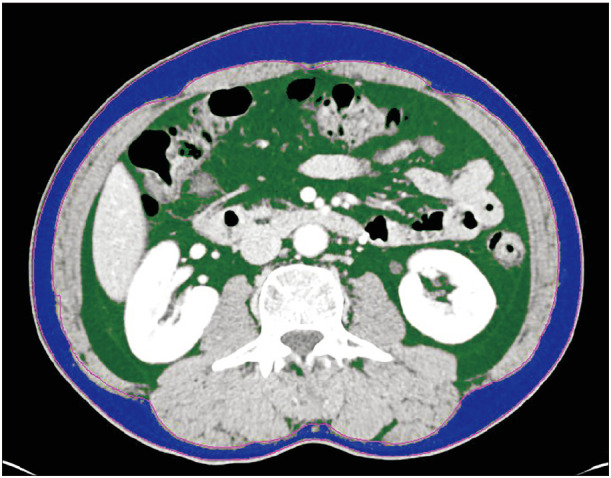
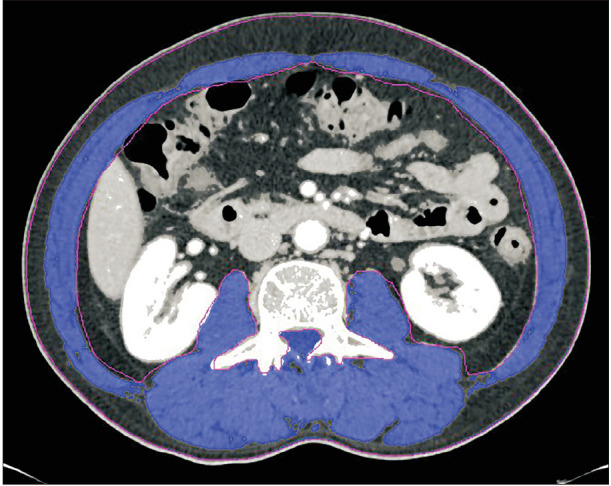
Contrast-enhanced abdominal CT scan was performed as routine staging work-up. One axial portal-phase image at the level of L3 was analyzed by a radiologist using commercial imaging software (TeraRecon Aquarius Workstation, TeraRecon, Foster City, CA, USA). According to the different Hounsfield units radiodensities of different tissue types, the software automatically calculates the skeletal muscle area (−29 to +150), abdominal fat area (−50 to −150), and subcutaneous fat area (−190 to −30) (Figs. 1 and 2) [14]. The SMI was calculated by dividing the muscle area (m2) by the square of the patient's height (cm2). Sarcopenia was defined as an SMI <41 cm2/m2 in women and <43 cm2/m2 in men with a body mass index (BMI) <25 kg/m2, and <53 cm2/m2 in men with a BMI >25 kg/m2 [15].
All patients were divided into 2 groups based on presence or absence of sarcopenia. Outcome measures included functional recovery parameters, length of hospital stay, postoperative complications, and survival data. Overall survival was defined as the time from surgery to death from any other causes or the last follow-up for alive status. Disease-free survival was defined as the time from surgery to the time of recurrence.
Statistical analysis was performed using IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA). The Mann-Whitney test was used for continuous parameters and a chi-squared test and/or Fisher exact test for categorical parameters were utilized for comparison of the differences between the 2 groups. Univariate logistic regression analyses for postoperative complications was performed to assess the influence on outcomes. Sex, BMI, sarcopenia, and laboratory parameters potentially predictive of postoperative complications were entered into multivariate logistic regression analyses. Univariate overall and disease-free survival rates were calculated using the Kaplan-Meier method, while multivariate overall and disease-free survival rates were calculated using Cox regression analysis. A P-value of <0.2 was adopted as the limit for inclusion of a covariant. A P-value of <0.05 was considered statistically significant.
Go to : 
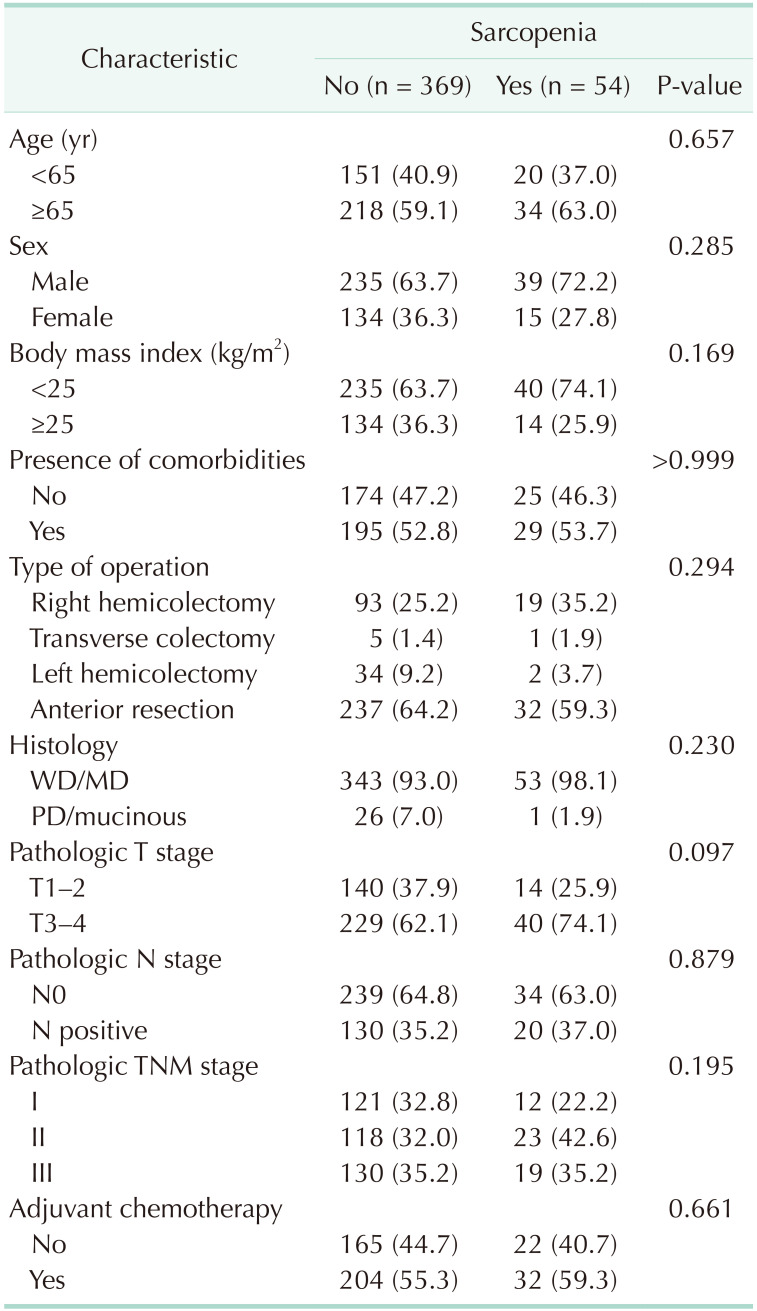
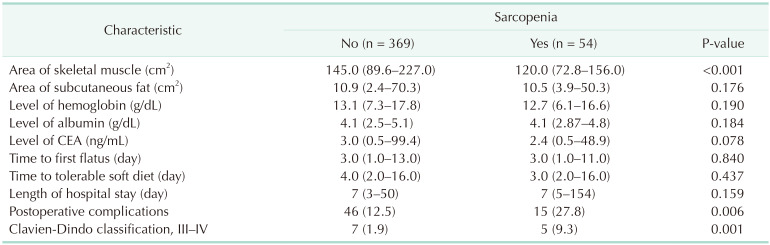
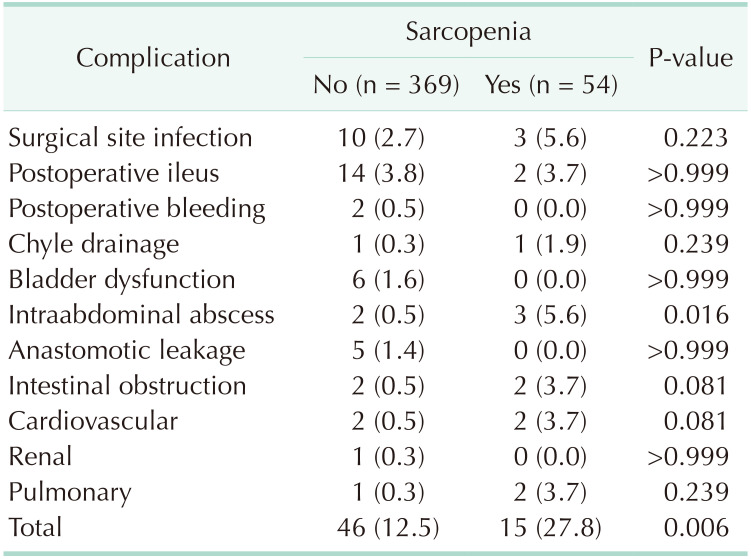
A summary of the demographics characteristics of patients with and without sarcopenia is shown in Table 1. Fifty-four patients had sarcopenia, while 369 patients did not. No significant differences were observed regarding age, sex, BMI, or presence of comorbidities between groups. Type of operation, histology, T and N stage, and pathologic stage were also similar between groups. Table 2 summarizes the differences in the variables between patients with and without sarcopenia. The skeletal muscle area was smaller in patients with sarcopenia, whereas the subcutaneous fat area was not different between groups in body composition analysis. It is noteworthy that functional recovery parameters, such as median time to first flatus (3 days), median time to tolerable soft diet (4 days), and median length of hospital stay (7 days) were not significantly different between the 2 groups. However, there were 46 patients (12.5%) without sarcopenia and 15 patients (27.8%) with sarcopenia who developed postoperative complications (P = 0.006). In particular, complications of Clavien-Dindo classification III were more frequent in patients with sarcopenia (P = 0.001). Types of complications according to presence or absence of sarcopenia are described in detail in Table 3.
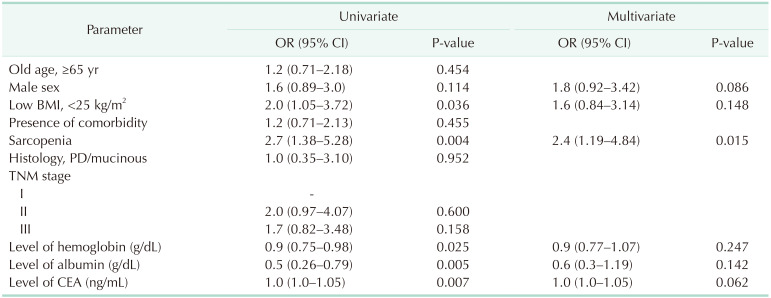
Table 4 identified potentially related factors predicting postoperative complications. Univariate logistic regression analyses showed that low BMI (P = 0.036), sarcopenia (P = 0.004), lower hemoglobin (P = 0.025), lower albumin (P = 0.005), and higher carcinoembryonic antigen level (P = 0.007) were significant factors associated with postoperative complications. Sarcopenia remained the most significant factor related to postoperative complications after multivariate regression analyses (P = 0.015).
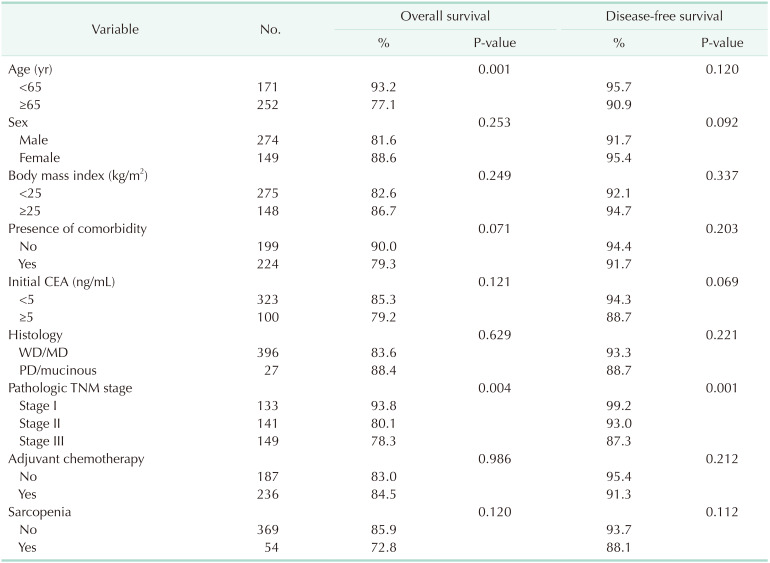
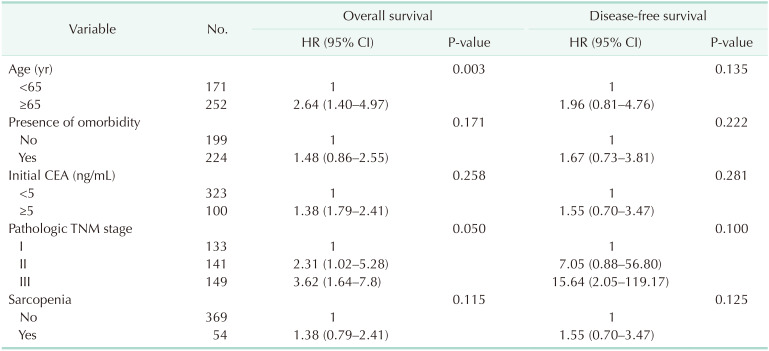
After a median follow-up of 48.7 months, the 5-year overall survival and disease-free survival rates, respectively, were in 85.9% and 93.7% in patients without sarcopenia, and 72.8% and 88.1% in patients with sarcopenia (P = 0.120 and P = 0.112, respectively). According to univariate analysis of factors affecting overall survival and disease-free survival, age (≥65 years) and pathologic TNM stage were significant prognostic factors for overall survival, while pathologic TNM stage was also a significant factor for disease-free survival (Table 5). Multivariate analysis of these factors showed that older age (≥65 years, P = 0.003) was a significant predictor for overall survival, while pathologic TNM stage showed a tendency to negatively affect overall survival (P = 0.050). There was no significant independent factor for disease-free survival (Table 6).
Go to : 
Previous studies regarding colorectal resection have shown that sarcopenia has a negative impact on postoperative morbidity and prolonged length of hospital stay after colorectal surgery [5678916]. However, these studies included mostly patients undergoing open resection. One previous study evaluated the impact of sarcopenia on laparoscopic surgery for colorectal cancer, and reported that there was no negative impact of sarcopenia on treatment results [13].
So far, prospective, randomized, controlled trials comparing open surgery and laparoscopic surgery for colon cancer have demonstrated that laparoscopic surgery is more advantageous for reduced hospital stay and fast recovery [121718]. Therefore, we postulated that laparoscopic surgery might eliminate the deleterious prognostic impact of sarcopenia with respect to not only short-term results, but also oncologic outcomes in patients with colon cancer. Indeed, in our study, sarcopenia did not affect functional recovery parameters (time to first flatus, time to tolerable soft diet, and length of hospital stay) after laparoscopic surgery. In particular, median length of hospital stay after surgery did not differ between patients with and without sarcopenia (7 days, P = 0.159). A similar study conducted by Pędziwiatr et al. [13] also showed that functional recovery after laparoscopic colorectal cancer surgery was similar regardless of presence or absence of sarcopenia. However, in our results, sarcopenia was revealed as an independent risk factor influenced postoperative complications (P = 0.015), even though demographic differences were not significant between groups. These results suggests that the amount of muscle of patients at the time of diagnosis is an important factor for short-term outcomes after laparoscopic surgery, whereas functional recovery may not be influenced by sarcopenia. Sarcopenia may associated with functional impairment and disability by affecting mobilization, impairing breathing, and decreasing physical performance [19]. The mechanism of sarcopenia is multifactorial. It can alter systemic inflammatory response, endocrine function, nutritional status, and insulin resistance [20]. These mechanisms may play a role increasing postoperative morbidity.
Although sarcopenia was negatively associated with postoperative complications, it did not affect oncologic outcomes after laparoscopic colon cancer surgery in our study. To the best of our knowledge, this is the first report to demonstrate the clinical impact of sarcopenia in patients with colon cancer undergoing laparoscopic surgery in terms of short-term and oncologic outcomes simultaneously.
Only few studies have described the association of sarcopenia with oncologic outcomes after colorectal cancer surgery [8]. Van Vledder et al. [21] reported that patients with sarcopenia showed worse survival than nonsarcopenic patients after resection of colorectal liver metastasis. Jung et al. [16] showed that the SMI had an adverse effects on mortality in older and obese patients who received adjuvant chemotherapy after colon cancer surgery. As patients with advanced stage of cancer should endure more challenging treatment, such as major hepatectomy and chemotherapy, those with sarcopenia might be associated with higher mortality. In addition, sarcopenia may reflect the increased metabolic activity of a more aggressive tumor biology, leading to systemic inflammation and muscle wasting [22]. These may explain why sarcopenia is a poor prognostic factor especially in advanced stage of disease. On the contrary, one study including patients with stage III colon cancer, instead of stage IV disease, showed that overall survival was not associated with sarcopenia [16]. In our study, excluding stage IV colon cancer, sarcopenia was not a significant prognostic factor for overall (P = 0.120) or disease-free (P = 0.112) survival. It can be inferred that sarcopenic patients who underwent laparoscopic surgery may not be affected by survival rate at relatively early stage of disease.
Routine preoperative CT has been suggested as a useful tool for body composition analysis [3515]. The prevalence of sarcopenia has been reported to range from 17.0% to 79% according to the cancer type and studied population [48]. It has been observed that patients with sarcopenia are in general older and have a low BMI [56]. The percentage of patients with sarcopenia in our study was 12.8%. The reason for the lower percentage compared with other studies may be the different value used for defining sarcopenia as well as the relatively healthy patients who could undergo laparoscopic surgery in our study.
The present study has several limitations, which are common for single-center, retrospective studies. First, we excluded patients with stage IV colon cancer. As we evaluated colon cancer treated by laparoscopic surgery and excluded cases of conversion to open surgery, these 2 conditions might have eliminated advanced-stage tumors. Further studies might be necessary to assess the impact of sarcopenia on short-term and/or oncologic outcomes after laparoscopic surgery including advanced-stage tumors such as other-organ invasive (T4) cancer and multiple liver metastases. Second, the definition of sarcopenia was different from other studies. We used cut-off values proposed by Martin et al. [15] for defining sarcopenia. However, different values may be used in other studies and this may influence the interpretation of the results [4].
The strength of our study is that we demonstrated the clinical impact of sarcopenia in patients with colon cancer undergoing laparoscopic surgery. As abdominal CT is routinely performed in staging work-up, CT-based body composition analysis is an efficient tool to assess a patient's function and frailty. Furthermore, we provided prognostic value in the Asian population, with a relatively large number of patients, even though cut-off values corresponding to Western populations were used in our study. More standardized measurement for body composition analysis based on CT scan is needed in the future.
In conclusion, patients with sarcopenia were not compromised functional recovery and median hospital stay after laparoscopic surgery for colon cancer although more frequent postoperative complications were observed. In addition, laparoscopic surgery may reduce the negative impact of sarcopenia on oncologic outcomes in colon cancer.
Go to : 
References
1. Milte R, Crotty M. Musculoskeletal health, frailty and functional decline. Best Pract Res Clin Rheumatol. 2014; 28:395–410. PMID: 25481423.
2. Cooper C, Dere W, Evans W, Kanis JA, Rizzoli R, Sayer AA, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012; 23:1839–1848. PMID: 22290243.
3. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008; 9:629–635. PMID: 18539529.
4. Levolger S, van Vugt JL, de Bruin RW, IJzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015; 102:1448–1458. PMID: 26375617.
5. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012; 107:931–936. PMID: 22871883.
6. Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015; 17:O256–O264. PMID: 26194849.
7. Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015; 17:O20–O26. PMID: 25328119.
8. Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol. 2015; 41:186–196. PMID: 25468746.
9. Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. . HPB (Oxford). 2011; 13:439–446. PMID: 21689226.
10. Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007; 25:3061–3068. PMID: 17634484.
11. Colon Cancer Laparoscopic or Open Resection Study Group. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009; 10:44–52. PMID: 19071061.
12. Clinical Outcomes of Surgical Therapy Study Group. Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004; 350:2050–2059. PMID: 15141043.
13. Pedziwiatr M, Pisarska M, Major P, Grochowska A, Matłok M, Przeczek K, et al. Laparoscopic colorectal cancer surgery combined with enhanced recovery after surgery protocol (ERAS) reduces the negative impact of sarcopenia on shortterm outcomes. Eur J Surg Oncol. 2016; 42:779–787. PMID: 27156809.
14. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985). 1998; 85:115–122. PMID: 9655763.
15. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013; 31:1539–1547. PMID: 23530101.
16. Jung HW, Kim JW, Kim JY, Kim SW, Yang HK, Lee JW, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer. 2015; 23:687–694. PMID: 25163434.
17. Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005; 6:477–484. PMID: 15992696.
18. Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of nonmetastatic colon cancer: a randomised trial. Lancet. 2002; 359:2224–2229. PMID: 12103285.
19. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002; 50:889–896. PMID: 12028177.
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010; 91:1123S–1127S. PMID: 20164314.
21. Van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012; 99:550–557. PMID: 22246799.
22. Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011; 62:265–279. PMID: 20731602.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download