Abstract
Purpose
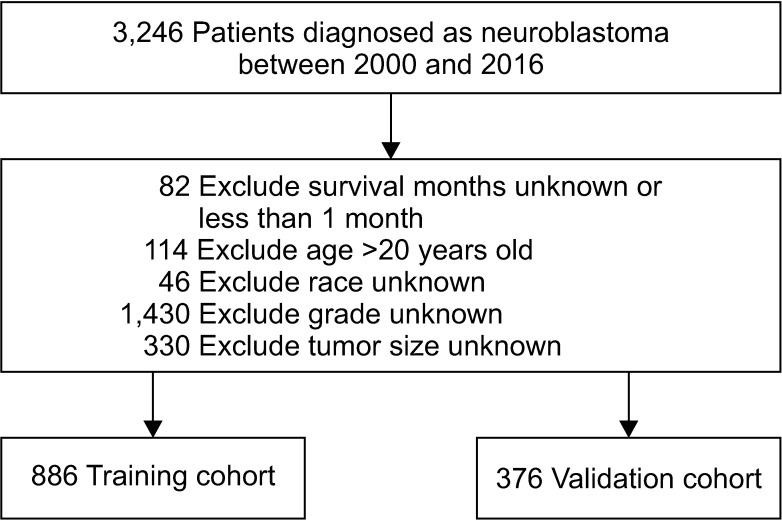
Methods
Results
Notes
Fund/Grant Support: We gratefully acknowledge the SEER database for allowing open access to the clinical data. This study was supported by Guangxi Natural Science Foundation Program (grant number 2014GXNSFAA118202) and Self-financing Scientific Research Project of Guangxi Zhuang Autonomous Region (grant number GZZC2019037).
References
Fig. 2
The influence of each independent predictive factor on overall survival by Kaplan-Meier survival analysis. (A–H) The survival curves of age, sex, race, radiation, grade, tumor size, tumor site, and chemotherapy, respectively. Annotation in (G): A, adrenal gland; B, soft tissue including heart; C, retroperitoneum; D, mediastinum and other respiratory organs; E, other.
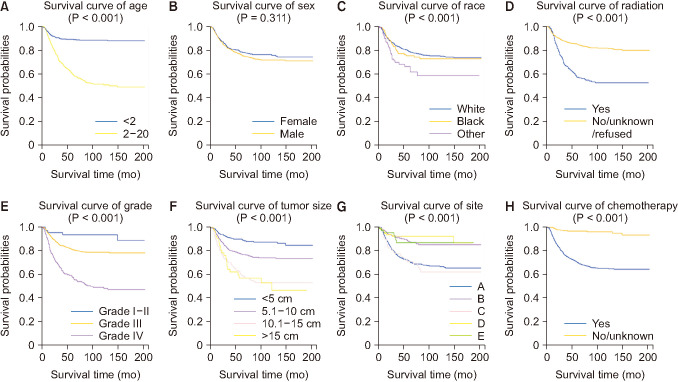
Fig. 3
The selection of predictive factors by least absolute shrinkage and selection operator (LASSO) regression. (A) LASSO coefficient profiles of the 8 variables. Predictors were chosen according to the minimum criteria, where optimal λ resulted in 8 nonzero coefficients. (B) The left and right dotted lines stand for the minimum criteria and 1 standard error criterion, respectively.
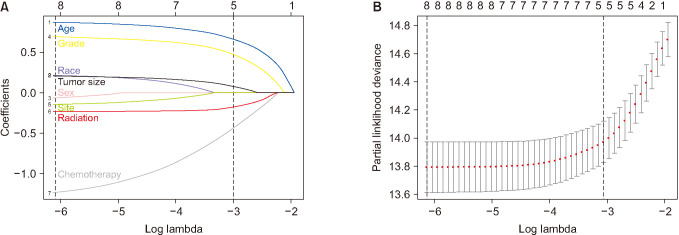
Fig. 4
(A) The nomogram of neuroblastoma. (B) The scores of an example. A, adrenal gland; B, soft tissue including heart; C, retroperitoneum; D, mediastinum and other respiratory organs; E, other.
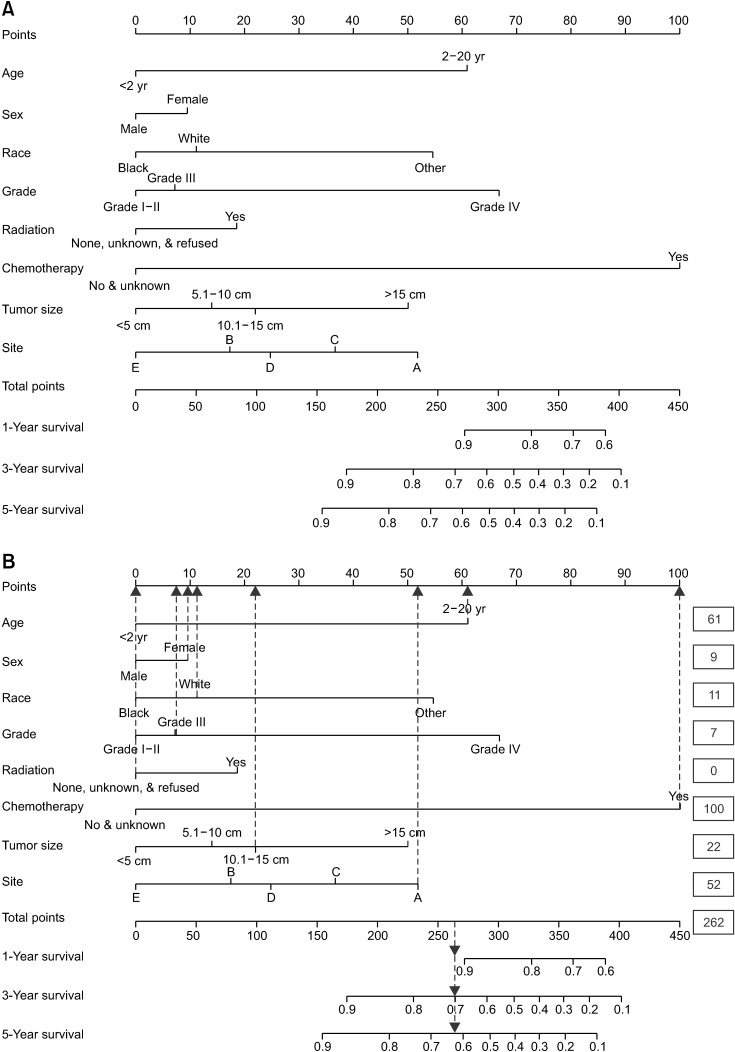
Fig. 5
The assessment of the predictive modelling by receiver operating characteristic curve. (A) Internal validation. (B) External validation. AUC, area under the curve.
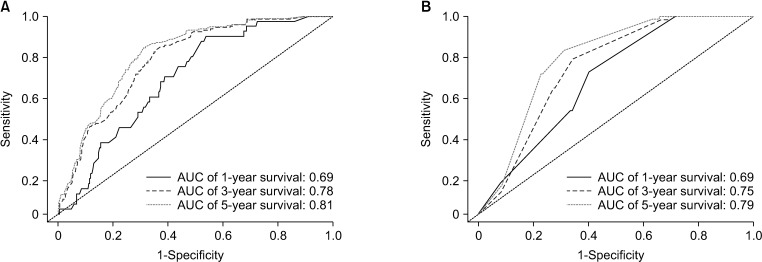
Fig. 6
The diagonal lines (dotted) were standard curves, and the colored lines were calibration lines of prediction. When the calibration lines get closer to the standard lines, the nomogram would have greater prognostic potential. (A) The calibration curves of internal validation for 1- , 3- , 5-year probabilities of survival. (B) The calibration curves of external validation for 1- , 3- , 5-year probabilities of survival.
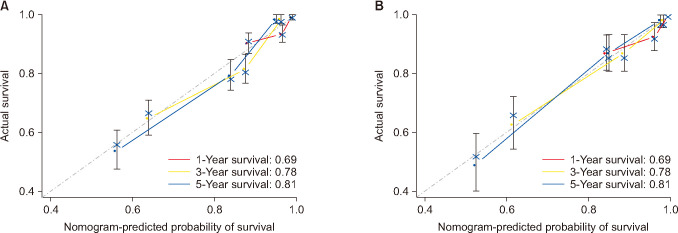
Table 1
Patient characteristics in the study
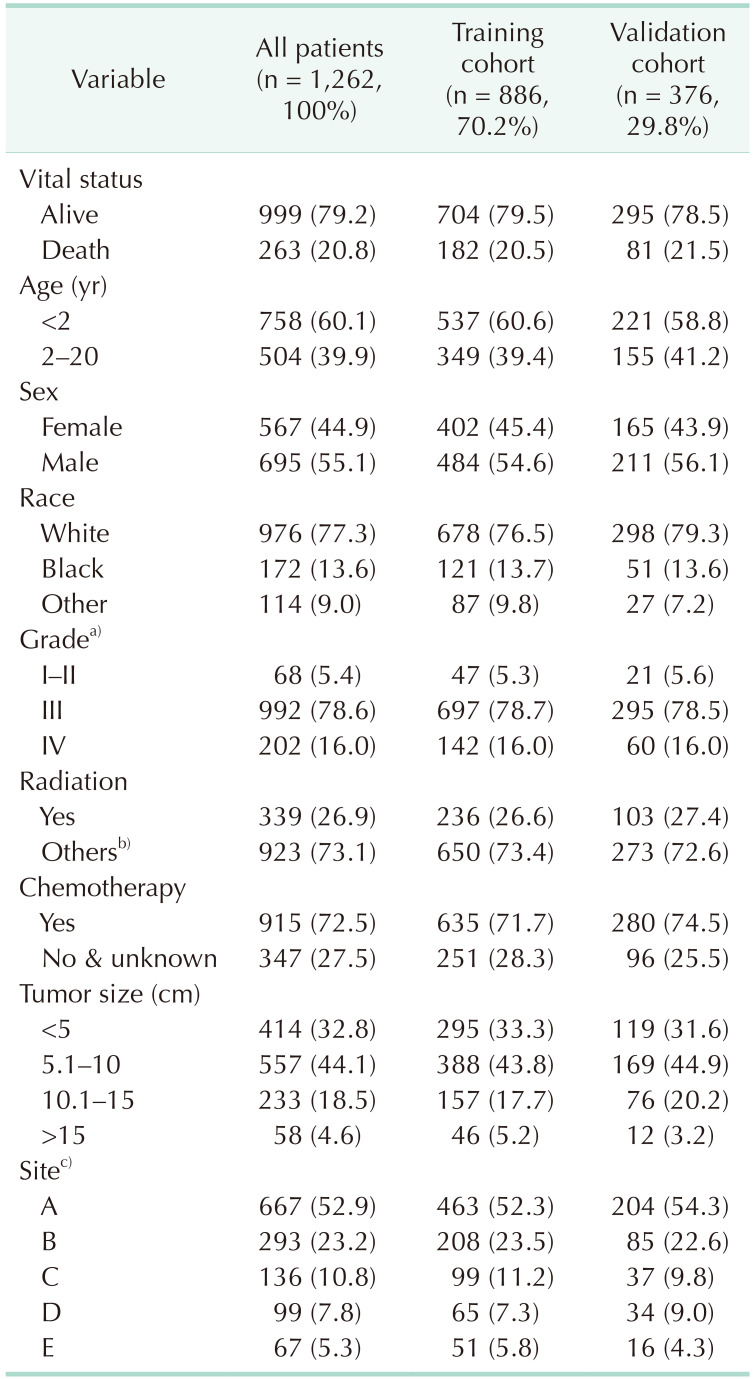
Values are presented as number (%).
a)Grade for differentiation of tumor cells: I–II, well differentiated and moderately differentiated; III, poorly differentiated; IV, undifferentiated/anaplastic. b)None, unknown, and refused. c)A, adrenal gland; B, soft tissue including heart; C, retroperitoneum; D, mediastinum and other respiratory organs; E, other.
Table 2
The numerical data (training cohort) used for developing the scores for each factor of nomogram
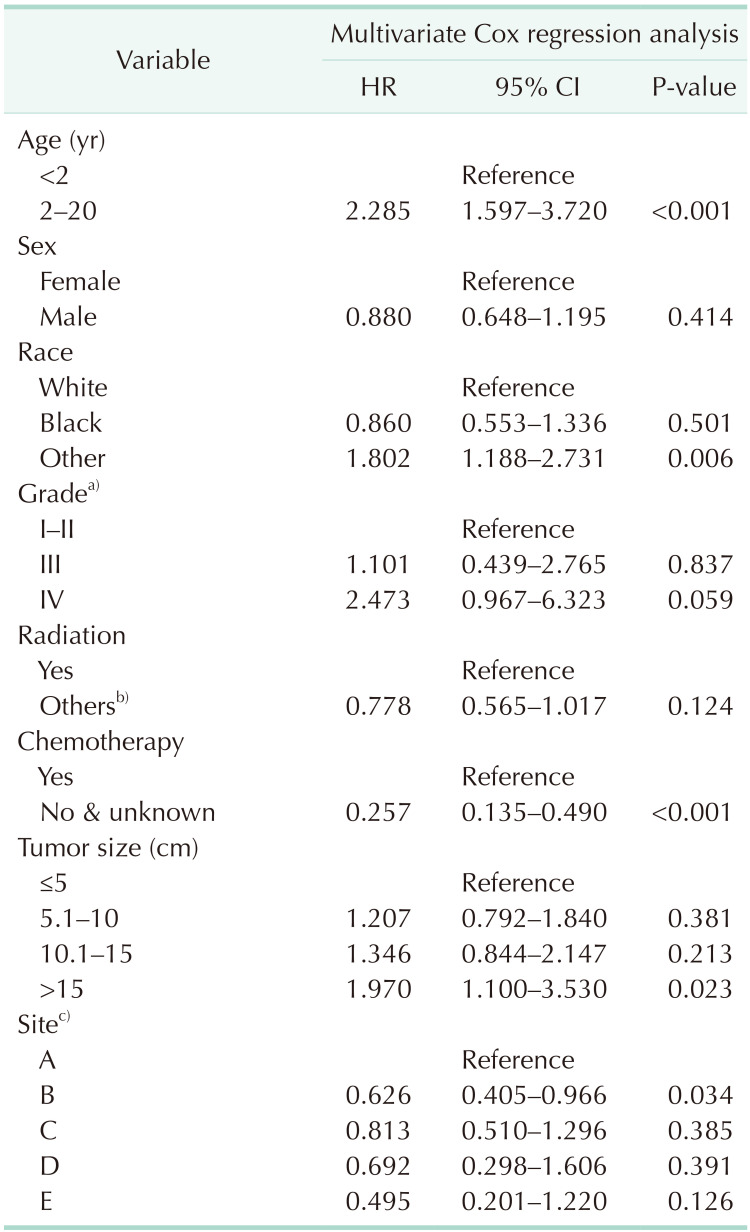
HR, hazard ratio; CI, confidence interval.
a)Grade for differentiation of tumor cells: I–II, well differentiated and moderately differentiated; III, poorly differentiated; IV, undifferentiated/anaplastic. b)None, unknown, and refused. c)A, adrenal gland; B, soft tissue including heart; C, retroperitoneum; D, mediastinum and other respiratory organs; E, other.
Table 3
Score assignment of the variables included in the nomogram
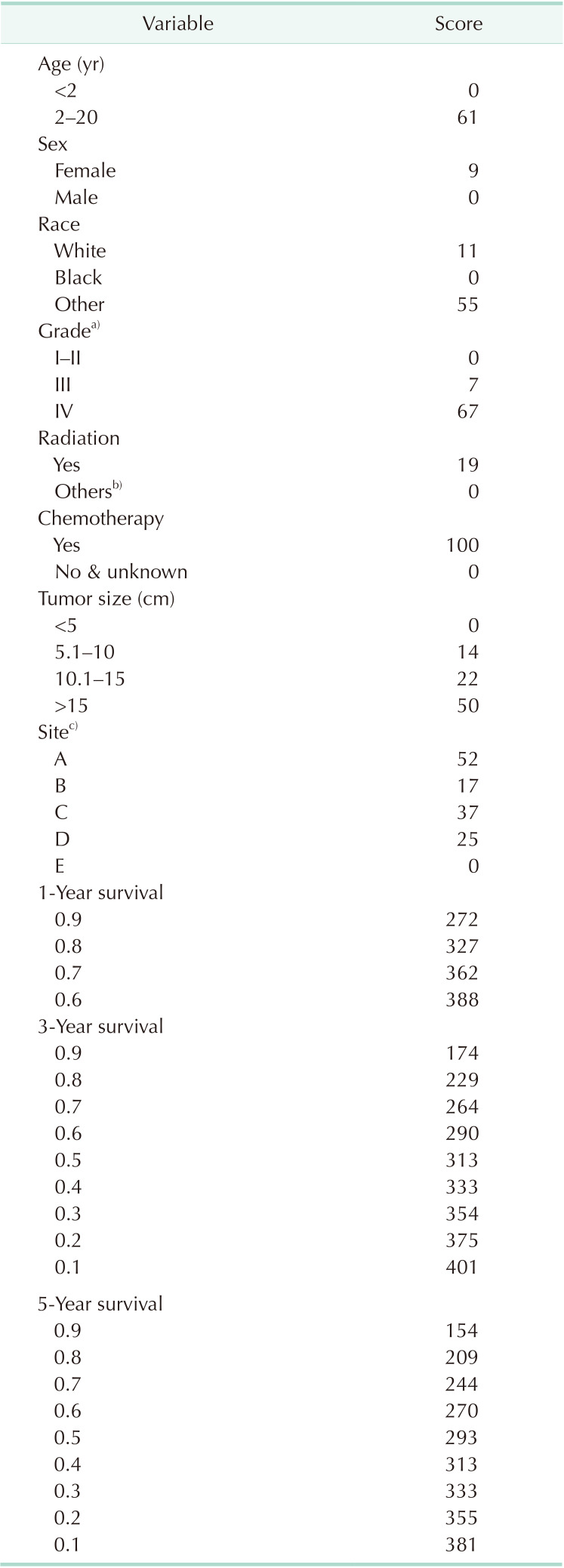
a)Grade for differentiation of tumor cells: I–II, well differentiated and moderately differentiated; III, poorly differentiated; IV, undifferentiated/anaplastic. b)None, unknown, and refused. c)A, adrenal gland; B, soft tissue including heart; C, retroperitoneum; D, mediastinum and other respiratory organs; E, other.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download