Abstract
Purpose
Retrorectal tumors (RTs) are a rare incidence and recommendations on the ideal surgical approaches are lacking. This study aimed to evaluate outcomes and follow-up results of patients undergoing excision of RTs at our institution.
Methods
A retrospective review was conducted for undergoing surgery for RT between January 2009 and January 2019. Demographic characteristics, presenting symptoms, preoperative diagnostic tests, surgical procedures, histopathological results, intraoperative and postoperative complications, postoperative hospital stay, postoperative 30-day mortality, 90-day unplanned readmission rate, and long-term outcomes were evaluated.
Results
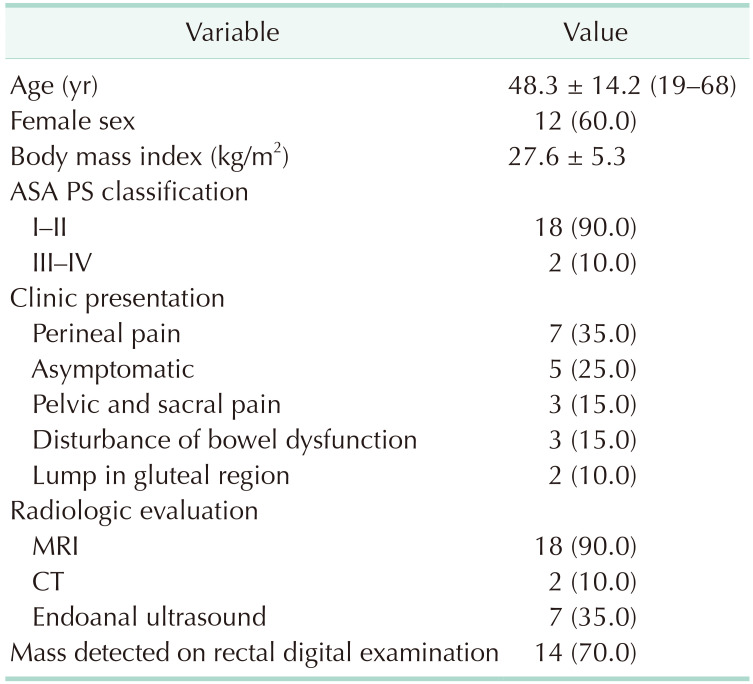
Twenty patients with a mean age of 48.3 ± 14.2 were analyzed. The most common presenting complaint was perineal pain (35.0%). Magnetic resonance imaging and computed tomography was preferred in 18 and 2 patients, respectively. Tumor localization was below the level of the third sacral vertebrae in 14 patients for whom the posterior surgical approach was used. No postoperative mortality was recorded at the end of follow-up of 53.8 ± 40 months. Mean length of postoperative hospital stay was 8.6 ± 9.4 days. Ten percent of the patients had unplanned hospital readmission within 90 days after discharge. Recurrence developed in 1 patient, for whom pathology were reported as chordoma.
Conclusion
RT should be managed by a multidisciplinary team given the complexity and heterogeneity of these tumors despite the fact that the majority are benign. A good understanding of pelvic anatomy and characterization of lesions through detailed radiological imaging is crucial to optimize surgical planning. Complete surgical resection is key for prolonged disease-free and overall survival of patients diagnosed with RTs.
Due to its rarity and asymptomatic nature, there is no clear information regarding the real prevalence of retrorectal tumors (RTs) [1]. Benign RTs mostly manifest as cystic followed by cysticsolid lesions, whereas malignant tumors contain predominantly solid components with necrotic areas and can invade surrounding tissue. Regardless of the benign or malign nature, the main target should be to remove the mass while intact with a negative surgical margin to avoid rupture and perforation during dissection. If these principles are not adhered to, most of these benign tumors will have local recurrence, and recurrent surgical interventions will be unavoidable.
Connectivity of nervous, skeletal, and intestinal systems during fetal life leads to formation of a diverse group of embryonal tumors in the rectorectal space, which is surrounded by the sacrum and the coccyx in the posterior, the mesorectum in the anterior, the Waldeyer's fascia below, and the peritoneal reflection above [2]. Histologic diversity of RTs renders their classification complicated; however, all primary tumors can be classified based on the predominant cell line type as congenital, neurogenic, osseous, inflammatory, or miscellaneous [3]. Limited data, predominantly limited to case reports, are available regarding management and long-term surgical outcomes of RT. Therefore, this study aimed to evaluate the outcomes and follow-up results of patients undergoing excision of RTs reflecting 10 years of experience at our institution.
Between 2009 and 2019, patients undergoing surgical excision for RT were analyzed through a prospectively maintained database at the Department of General Surgery in Çukurova University Faculty of Medicine (Adana, Turkey). This retrospective study was approved by the Institutional Review Board of Çukurova University (No. 86/24, dated 08.03.2019.) Patient files and hospital information system records were analyzed retrospectively and the study was designed by creating a prospective database. Follow-up of the patients was provided by electronic records, office visits, and telephone connection. Patients diagnosed with primary or recurrent rectal cancers, urogynecologic tumors, and retrorectal abscess were excluded from the study.
Patients' demographic characteristics, body mass index, American Society of Anesthesiologist (ASA) physical status classification, admission symptoms, diagnostic methods, details regarding procedures performed, intraoperative and postoperative complications (Clavien-Dindo classification) [4], postoperative hospital stay, postoperative 30-day mortality, 90-day unplanned readmissions, mean follow-up, recurrence, and histopathological findings were recorded. As a part of our clinical strategy, preoperative biopsy was not planned due to the concern of tumor seeding and other potential complications including surgical site infection.
Patients were informed about the surgical procedure and written informed consent was obtained. Complete bowel cleansing was performed in all cases. Prophylaxis for venous thromboembolism was provided with a low molecular weight heparin one night before surgery and continued for up to 4 weeks after discharge for patients who had undergone pelvic surgery. Antibiotic prophylaxis was delivered with 500-mg metronidazole 30 minutes before surgery and 1-g cefazolin before induction. All operations were performed under general anesthesia. A ureter catheter was placed preoperatively in cases where the mass was found to be close to the ureter.
Surgical planning was determined based on several factors. These included the location of the tumor (above or below the level of the third sacral vertebrae), the relationship of the tumor with the surrounding structures (sacrum, pelvic sidewall, and internal organs), and the potential for malignancy of the tumor. Considering these factors, 1 of the 3 different surgical approaches (anterior, posterior, or combined) was chosen. The posterior approach was preferred for the masses located below the third sacral vertebral level, and the anterior (abdominal) approach was preferred for the masses above this level. The combined (anterior-posterior) approach was preferred for the masses located below the level of the third sacral vertebrae but extending above this level, or if there is an invasion to the surrounding organs.
In the anterior approach, surgical technique (laparoscopic or open) was chosen depending on the surgeon's preferences. Laparoscopic procedures performed with 4 trocars; a 12-mm trocar was inserted 2 cm above from umbilicus for the camera, one 5-mm trocar was inserted into the right upper quadrant, one 5-mm trocar for right lower quadrant, and one 5-mm trocar for the left upper quadrant. The patient was placed in a head-down position with a slight tilt to the left side. For both laparoscopic and open technique, the same surgical steps were followed as follows; sigmoid colon was mobilized and embryological plans were entered in front of the promontorium. The mass was reached by moving between anterior mesorectum and posterior presacral fascia with particular respect to vascular structures within the pelvis, mesorectum integrity, ureters, and pelvic autonomic nerves and then excised completely via a small Pfannenstiel incision.
In the posterior approach (Kraske procedure), patients were placed in the jackknife position and the incision through the midline coccyx tip was preferred. The patient's buttocks were taped to the side of the table for better exposure. The retrorectal cavity was reached by cutting the anococcygeal ligament. During the dissection of the rectal wall, bimanual examination was performed with rectal touch when necessary and the margins of the mass and rectal wall were separated. If necessary, coccyx resection was added to the surgical specimen. All wounds were closed in layers and negative suction drains were used when necessary.
In the combined approach, these 2 techniques (first anterior, then posterior approach) were applied together and open technique was preferred as an anterior approach. Retrorectal distance was reached with the anterior approach, then the mass was released by thin and sharp dissections from surrounding tissues and structures (bladder and pelvic sidewall vessels). This dissection was extended to the level of the levator ani muscle, then the abdomen was closed and the patient placed in the prone jackknife position for posterior approach. If sacrectomy was planned, after rectal mobilization, the presacral venous plexus was ligated as well as the internal iliac artery-vein, including sacral nerve roots if necessary. Presacral soft tissue was dissected to make anterior osteotomies from suitable surgical margins, which was already decided from previous radiologic studies under fluoroscopic control. The abdomen was closed after the presacral area was covered with gauze and the patient placed in the prone position. Wide skin and soft-tissue resection were made to achieve negative margins when the tumor reached subcutaneous tissue. Laminectomy was created from a tumor-free margin to keep the suitable sacral roots alive and to ligate the others. Suitable osteotomies were done to complete partial sacral transection and the tumor removed en bloc. This part of the surgery was completed by the orthopedic oncologist-spine surgeon. Cutaneous and/or myocutaneous flaps were preferred to cover the defect area in patients with extensive skin removal. Negative-pressure closed-system drains were placed under the flap.
Data were analyzed using IBM SPSS Statistics for Windows, ver. 24.0 (IBM Corp., Armonk, NY, USA). Categorical measurements were summarized as numbers and percentages, and continuous measurements were summarized as mean ± standard deviation (median and range where necessary).
Twenty patients (12 female and 8 male) with a mean age of 48.3 ± 14.2 years were analyzed. The body mass index of the patients was 27.6 ± 5.3 kg/m2. The most common presenting complaint was perineal pain (35.0%). Digital rectal examination (DRE) was performed to each patient, a palpable mass was detected in 15 patients (75.0%). Four patients were asymptomatic and the mass was found incidentally. MRI and CT were preferred in 18 and 2 patients, respectively. The specificity of MRI was 87.5%. CT angiogram was also used in 2 patients to reveal the arterial vascular structure of the mass in order to reduce bleeding during surgery. Endoanal ultrasound was performed for further anatomical delineation in 7 patients. Fig. 1 represents radiologic images for a patient diagnosed with chordoma. Demographic characteristics and details regarding clinic and radiologic evaluation are presented in Table 1.
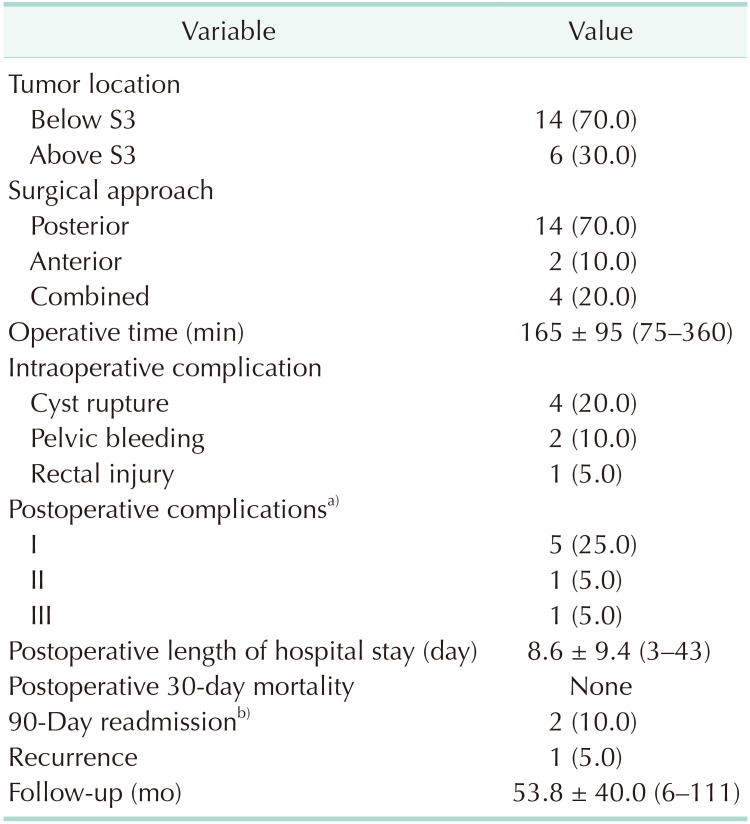
Three different approaches were applied as surgical treatment and surgical method was determined based on the tumor location. Tumor localization was below the level of the third sacral vertebrae in 14 patients for whom the posterior surgical approach was implemented (Fig. 2). Anterior and combined approach were applied in 2 and 4 patients, respectively. The laparoscopic technique was chosen as an anterior approach in 1 patient. Radiologic imaging, operative view, and extracted specimen of that patient are shown in Fig. 3. The combined approach for the chordoma patient is illustrated in Fig. 4. None of the cases managed with a combined approach required additional organ resection or diverting/end colostomy. Myocutaneous flap was required in 2 patients who were diagnosed with chordoma and treated with a combined approach.
The mean operation time was 165 ± 95 minutes (range, 75–360 minutes). The most common intraoperative complication was mass rupture (n = 4) followed by pelvic hemorrhage (n = 2) and rectal wall perforation (n = 1). Data regarding operative procedures and postoperative outcomes are presented in Table 2. No postoperative mortality was recorded. Postoperative complications based on the Clavien-Dindo classification were as follows: grade I, 25.0%; grade II, bleeding requiring transfusion (5.0%); and grade III, wound infection requiring intervention (5.0%). Intraoperative hemorrhage originating from the presacral venous plexus was controlled with a temporary tamponade followed by application of a procoagulant chemical.
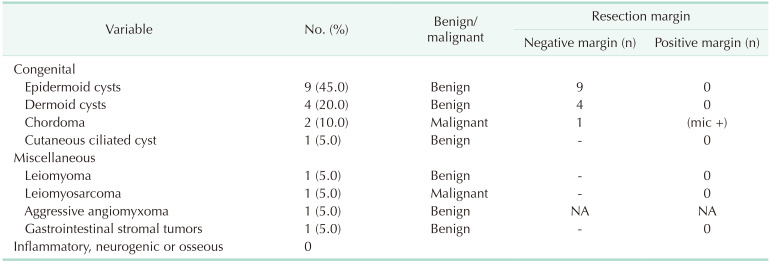
The mean tumor diameter was 5.4 ± 2.6 cm. Based on the Uhlig and Johnson classification, congenital tumors were detected in 80.0% of our patients, and miscellaneous tumors were found in the remaining 20%. In the malignant subgroup, chordoma and leiomyosarcoma were reported in 2 and 1 patients, respectively. The rest of all patients were benign and internal epidermoid cyst was most common. The R0 resection rate was 95.0% in our series. Resection margins were positive in 1 patient diagnosed with chordoma. Local recurrence was detected at the 1-year follow-up in this particular patient. Histopathologic findings are summarized in Table 3.
Postoperative hospital stay was 8.6 ± 9.4 days (range, 3–43 days). The rate of unplanned 90-day readmission was 10% (n = 2). Reasons for readmission were wound infection leading to sinus formation and breakdown of the skin flap. At the end of follow-up of 53.8 ± 40 months, 1 chordoma patient experienced postoperative sexual dysfunction and recurrence.
The main findings of this series of 20 cases were that the exact anatomic delineation through MRI and endoanal ultrasound work-up is required in order to obtain improved surgical outcomes of RT and that a significant number of resections can be performed by avoiding an abdominal approach with a low morbidity rate. Achieving R0 resection should be the primary goal for favorable long-term outcomes.
Knowledge of anatomical structures surrounding the retrorectal space is of paramount importance. The anatomical region in this complex structure contains multiple embryological residues and can host many different tumor types [2]. Congenital lesions are the most common presacral retrorectal lesions constituting the majority of all lesions. These originate from embryological residues, are usually benign, and more common in women, as correlated with our series. The most common subtypes are developmental cysts, chordomas, and anterior meningoceles [25]. Developmental cysts account for 60% of all congenital presacral lesions and may originate from any of the 3 embryonic germ layers. They are classified as epidermoid, dermoid, tailgut, or teratomas according to the germ layer they originate from. Developmental cysts have high secondary infection rates when ruptured intraoperatively [567]. Epidermoid and dermoid cysts are caused by abnormal closure of the ectodermal tube. While epidermoid cysts are benign unilocular lesions composed of monolayer squamous cells, dermoid cysts contain not only monolayer squamous cells but are also more mature elements such as hair follicles, dermal attachments, and sweat glands [56]. In our series, developmental cysts constituted 80% of congenital lesions. Cyst rupture occurred in 2 patients but no postoperative complication was observed, even in the long-term.
Complete and nonpiecemeal excision of all RT is critical to obtain favorable surgical outcomes considering that ‘other’— which refers to malignant miscellaneous and metastatic—tumors account for 10%–20% of all RT. In the most extensive review article in the literature, 341 studies including 1,708 patients were evaluated. Benign pathologies were the most common lesions and congenital tumors were the most commonly reported histopathologic type with a rate of 70%. In this study, ‘other’ tumors of the retrorectal area (fibroma, leiomyoma, hemangioma, endothelioma, liposarcoma, fibrosarcoma, malignant histiocytoma, leiomyosarcomas, hemangiopericytoma, and metastatic adenocarcinoma) constituted 19.1% of total patients [7]. In our study, ‘other’ tumors were leiomyoma, leiomyosarcoma, aggressive angiomyxoma, carcinoma, and gastrointestinal stromal tumors; and these accounted for around 20% of all patients.
DRE is a simple and easy adjunctive diagnostic method in the diagnosis and treatment of pelvic tumors. It may provide important information in predicting the location of the tumor in the presacral region, and in determining the histological structure and boundaries of the tumor, but this does not apply to every retrorectal tumor [8]. Baek et al. [9] emphasized that in their series, DRE was able to detect the mass in 90% of patients. In our study, this rate only reached 70%. In tumors with more aggressive patterns and clinical behavior, DRE alone cannot be a sufficient diagnostic method to evaluate the tumor. In this case, the relationship between the tumor and the surrounding tissue should be clearly determined by supportive imaging methods.
The most common imaging modalities are CT and MRI [1011]. MRI is preferred over CT because it predicts benign or malignant characteristics of the tumor. Hopper et al. [10] emphasized that MRI is superior to CT (94% vs. 64%) in differentiating benign or malignant potentials of the tumor. Similar results were reported by the study of Chereau et al. [11]. Endoanal ultrasonography is another imaging method used to identify the relationship of RTs with the rectal wall and other adjacent structures [11]. In our series, endoanal ultrasonography was preferred in only 35.0% of the patients to delineate the RT related anatomical plans.
Debate exists over the role of preoperative biopsy in the diagnosis and management of RT. Many authors do not recommend preoperative biopsy due to the risk of tumor spread to different planes, bleeding, and the potential for infection [121314]. However, there are also supporters of the opposing view. Merchea et al. [15] found that the preoperative biopsy had 100% specificity and 96% sensitivity in distinguishing the diagnosis of benign or malignant disease, and emphasized that preoperative biopsy was safe and allowed more optimal management of treatment. As another result of this study, the specificity of distinguishing between benign or malignant disease by imaging method was 81% and sensitivity was 83% [15]. In another study, it was concluded that it could be helpful in the diagnosis of Ewing's sarcoma, neurofibrosarcoma, or desmoid tumor, which may receive benefits from preoperative biopsy and neoadjuvant therapy [1016]. More importantly, a careless transrectal needle biopsy of meningocele can cause undesirable consequences such as meningitis, and even subsequent death [17]. As can be seen, there is still controversy surrounding preoperative biopsy for RT. However, the only thing emphasized as a common interpretation by advocates of both views is that transrectal or transvaginal biopsy should never be performed. Additionally, it is obvious that such biopsy procedures may cause malignant lesions to spread, or cause needle line transplantation. Considering that the main target of the surgical treatment is not to leave any tumors behind, preoperative biopsy does not prove utility in the RT management and does not change the management. Since the biopsy line has to be removed with the tumor as a whole, for example, transrectal biopsy of the sample will necessitate proctectomy in a patient whose rectum can be preserved. In the presence of a cystic lesion, if an approach like this results in an infection, this could lead to an increased risk of postoperative complications and recurrence. In our clinical practice, we do not undertake preoperative biopsy, especially after characterization of the lesion with a high-quality MRI.
Chordomas, as the most common malignant tumor among retrorectal masses, are very aggressive tumors with high recurrence rates and low survival rates even after radical surgery [2]. These tumors are thought to be caused by primitive notochord extending embryologically from the occiput base to the caudal border in the embryo. These tumors can be located anywhere along the spine; however, most prefer the sacrococcygeal region. Despite slow growth patterns, these tumors progress by invading surrounding tissues, and nearly 20% of chordomas have distant metastases [18]. Bosca et al. [2] reported in their study that chordoma was the most common malignant tumor group, with 88.7% of the pathologically diagnosed malignant tumors being chordomas. Half of the chordomas in this report had to be reoperated due to local-regional recurrence and the 5-year survival rate was only 38.7% [2]. In another study involving 52 sacrococcygeal chordoma cases, 44% of local recurrence was detected [19]. In another study, 10-year survival for chordomas, despite complete radical resection, was reported to be only 9%–35% [16].
Surgical resection is the mainstay of therapy for chordoma. However, complete resection is often not possible because of the invasion of surrounding tissues in patients with advanced disease. Therefore, surgical treatments always require en-bloc wide excisions. However, when complete resections are difficult to achieve, tumor recurrence is inevitable. There are no randomized, controlled trials of chordoma that proved any clinical benefit for systemic chemotherapy. Adjuvant radiotherapy is therefore suggested for most chordoma patients to control local recurrence. In the last decade, a significant antitumor effect has been demonstrated with imatinib, a tyrosine kinase inhibitor, for the treatment of advanced chordoma disease. If the disease progresses after imatinib administered alone, then the combination with cisplatin may be used as a second-line treatment [2021]. Unfortunately, there is still lack of data for complementary therapies (chemotherapy, chemotherapy combined with radiotherapy) for soft-tissue sarcomas; therefore, surgical treatment with wide negative margins is the most important factor for prolonged overall survival [22]. In our series, the pathological diagnosis of 3 patients treated with combined surgical approach was reported as malignant disease: 2 chordomas and 1 leiomyosarcoma. The surgical margin was microscopic positive in 1 chordoma patient and tumor recurrence was observed at the one-year follow-up. This patient was managed with debulking surgery followed by radiotherapy. Adjuvant chemotherapy treatment was not given to any malignant patients after discussion among the multidisciplinary tumor council.
RT presents a multitude of challenges to surgeons, especially for those uninitiated in pelvic surgery. The exact localization of the tumor and its relationship with surrounding structures must be clearly demonstrated in order to create a viable surgical plan. The type of preferred surgical approach depends on the location, size of the tumor, the relationship between the highest upper cranial (proximal) border reached, especially in the presacral region, and the surrounding organs. Understanding the extent of the resection field is the most critical point of preoperative planning. Collaborative efforts with a multidisciplinary team of radiologist, pathologist, medical oncologist and radiation oncologist, and colorectal, orthopedic, plastic, urologic, neurosurgical surgeon is a prerequisite in case of invasion of adjacent anatomical structures.
Our study carries limitations including the low patient numbers and retrospective design. Considering the rare incidence of the disease, further research with a multicentric prospective design may be beneficial to better determine the natural course of the disease in the long-term.
RT should be managed by a multidisciplinary team given the complexity and heterogeneity of these tumors despite the majority being benign. A good understanding of pelvic anatomy and characterization of lesion through detailed radiological imaging is crucial to optimize surgical planning. For lesions not extending above the level of third sacral vertebrae, a posterior approach can be safely performed with low morbidity and recurrence rate in the long-term.
References
1. Jao SW, Beart RW Jr, Spencer RJ, Reiman HM, Ilstrup DM. Retrorectal tumors: Mayo Clinic experience, 1960-1979. Dis Colon Rectum. 1985; 28:644–652. PMID: 2996861.
2. Bosca A, Pous S, Artes MJ, Gomez F, Granero Castro P, Garcia-Granero E. Tumours of the retrorectal space: management and outcome of a heterogeneous group of diseases. Colorectal Dis. 2012; 14:1418–1423. PMID: 22390258.
3. Uhlig BE, Johnson RL. Presacral tumors and cysts in adults. Dis Colon Rectum. 1975; 18:581–589. PMID: 1181162.
4. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213. PMID: 15273542.
5. Hobson KG, Ghaemmaghami V, Roe JP, Goodnight JE, Khatri VP. Tumors of the retrorectal space. Dis Colon Rectum. 2005; 48:1964–1974. PMID: 15981068.
6. Hassan I, Wietfeldt ED. Presacral tumors: diagnosis and management. Clin Colon Rectal Surg. 2009; 22:84–93. PMID: 20436832.
7. Abel ME, Nelson R, Prasad ML, Pearl RK, Orsay CP, Abcarian H. Parasacrococcygeal approach for the resection of retrorectal developmental cysts. Dis Colon Rectum. 1985; 28:855–858. PMID: 4053899.
8. Bouts C, Van der Speeten K. A single center retrospective analysis of Kraske’s transsacral approach: a review. Surg Sci. 2014; 5:454–466.
9. Baek SK, Hwang GS, Vinci A, Jafari MD, Jafari F, Moghadamyeghaneh Z, et al. Retrorectal tumors: a comprehensive literature review. World J Surg. 2016; 40:2001–2015. PMID: 27083451.
10. Hopper L, Eglinton TW, Wakeman C, Dobbs BR, Dixon L, Frizelle FA. Progress in the management of retrorectal tumours. Colorectal Dis. 2016; 18:410–417. PMID: 26367385.
11. Chereau N, Lefevre JH, Meurette G, Mourra N, Shields C, Parc Y, et al. Surgical resection of retrorectal tumours in adults: long-term results in 47 patients. Colorectal Dis. 2013; 15:e476–e482. PMID: 23601092.
12. Lev-Chelouche D, Gutman M, Goldman G, Even-Sapir E, Meller I, Issakov J, et al. Presacral tumors: a practical classification and treatment of a unique and heterogeneous group of diseases. Surgery. 2003; 133:473–478. PMID: 12773974.
13. Macafee DA, Sagar PM, El-Khoury T, Hyland R. Retrorectal tumours: optimization of surgical approach and outcome. Colorectal Dis. 2012; 14:1411–1417. PMID: 22339762.
14. Sagar AJ, Tan WS, Codd R, Fong SS, Sagar PM. Surgical strategies in the management of recurrent retrorectal tumours. Tech Coloproctol. 2014; 18:1023–1027. PMID: 24925354.
15. Merchea A, Larson DW, Hubner M, Wenger DE, Rose PS, Dozois EJ. The value of preoperative biopsy in the management of solid presacral tumors. Dis Colon Rectum. 2013; 56:756–760. PMID: 23652750.
16. Neale JA. Retrorectal tumors. Clin Colon Rectal Surg. 2011; 24:149–160. PMID: 22942797.
17. Ucar AD, Yoldas T, Oymaci E, Erkan N, Caliskan C, Yildirim M, et al. Totally curative surgical resection of retrorectal tumors. Hepatogastroenterology. 2015; 62:606–611. PMID: 26897938.
18. Woodfield JC, Chalmers AG, Phillips N, Sagar PM. Algorithms for the surgical management of retrorectal tumours. Br J Surg. 2008; 95:214–221. PMID: 17933000.
19. Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005; 87:2211–2216. PMID: 16203885.
20. Stacchiotti S, Longhi A, Ferraresi V, Grignani G, Comandone A, Stupp R, et al. Phase II study of imatinib in advanced chordoma. J Clin Oncol. 2012; 30:914–920. PMID: 22331945.
21. Casali PG, Stacchiotti S, Grosso F, Messina A, Crippa F, Tamborini E, et al. Adding cisplatin to imatinib re-establishes tumor response following secondary resistance to imatinib in advanced chorcoma (abstract 10038). J Clin Oncol. 2007; 25:554s.
22. Lee SE, Park SY. Sarcomatoid carcinoma of the small intestine: a rare and highly aggressive tumor. J Korean Surg Soc. 2012; 83:321–324. PMID: 23166892.
Fig. 1
(A) CT shows soft-tissue (white arrow) mass with an approximately 8.5 × 7.0-cm axial diameter destructing the sacrum, containing coarse calcifications. (B) MRI shows a mass of 9.0 cm × 8.4 cm × 8.3 cm in the posterior of the sacrum with a lobulated contoured heterogeneous chondrogenic matrix (white arrow).
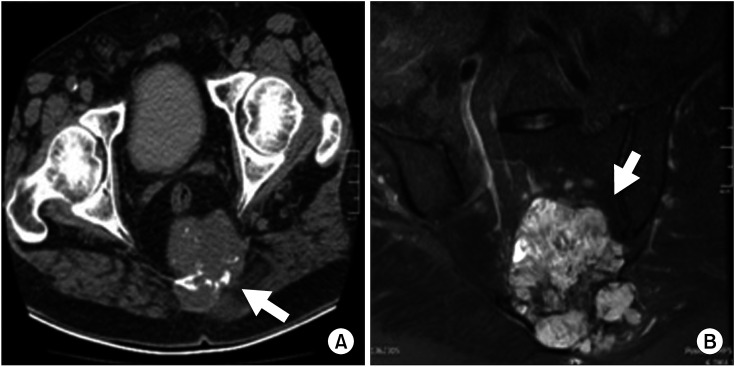
Fig. 2
Posterior approach. (A) T1-weighted fat sat contrast-enhanced MRI is indicating the homogeneous cystic lesions at the tip of the coccyx (white arrow). (B) Incision through the midline coccyx. (C) The surgical specimen, cyst resection with coccyx.
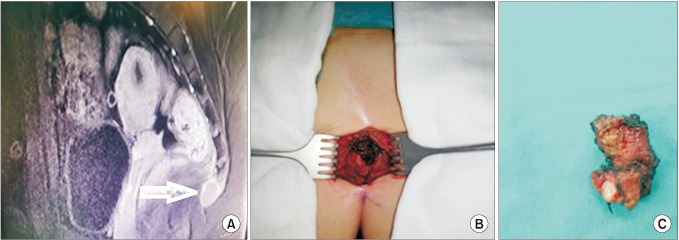
Fig. 3
Anterior approach. (A) MRI shows the soft-tissue mass in the posterolateral part of the rectum, probably considered to be of mesenchymal origin in 9.0 × 7.0 × 7.0-cm dimensions partially extending to the ischial fossa. (B) Laparoscopic view of retrorectal space (white arrow) and mesenchymal mass (black arrow). (C) A view of soft-tissue mass after laparoscopic resection.
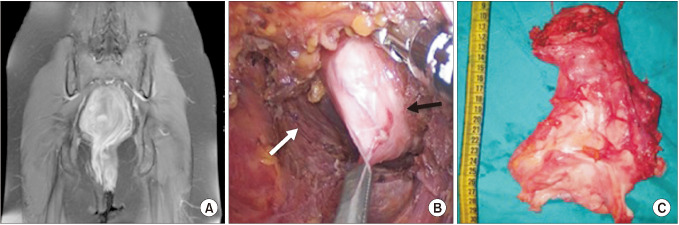
Fig. 4
Combined approach. (A) Anterior view of the chordoma (black arrow) in retrorectal area. (B) Posterior view after partial sacrectomy. Black arrow shows resection margin of the partial sacrectomy and white arrow shows posterior face of mesorectum. (C) Right gluteus maximus myocutaneous perforator flap was designed with 15.0 × 8.0-cm skin part and the flap was rotated 110° to the sacral defect area. (D) The donor area was closed primarily and the flap was fully adapted onto the sacral area.
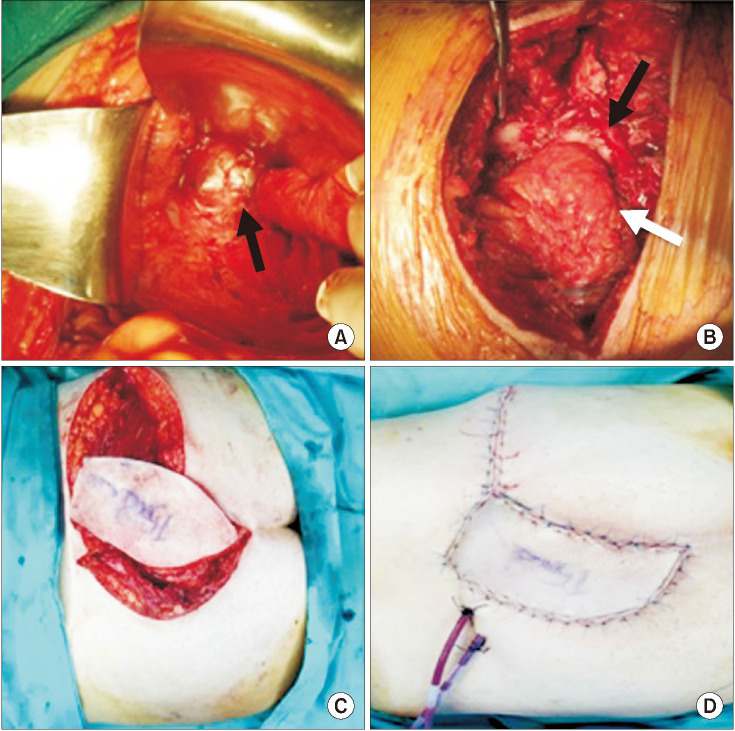
Table 1
Demographic characteristics and details regarding clinic and radiologic evaluation of retrorectal tumor patients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download