Abstract
Purpose
Klotho is an antiaging factor mainly produced by renal tubular cells. Klotho is reportedly decreased in an animal model of acute kidney injury and patients with chronic kidney disease. However, information on Klotho expression after kidney transplantation is limited. We analyzed the correlation between donor Klotho expression and clinical outcomes of kidney transplantation.
Methods
Sixty patients who underwent deceased donor kidney transplantation between March 2015 and October 2017 were enrolled. Serum and tissue Klotho expression levels were measured by enzyme-linked immunosorbent assay and immunohistochemistry, respectively. Graft function was assessed by estimated glomerular filtration rate (eGFR).
Results
Patients were divided into 2 groups according to donor Klotho expression in renal tissues. A greater improvement in eGFR was observed at 1 week after transplantation in patients receiving kidneys with higher Klotho expression (47.5 ± 21.9 mL/min/1.73 m2
vs. 63.9 ± 28.2 mL/min/1.73 m2, P = 0.030). Patients were also classified into 2 groups according to donor serum Klotho level. There was a tendency for a higher eGFR at 12 months after transplantation in patients receiving kidneys from donors with a higher Klotho level (51.0 ± 18.0 mL/min/1.73 m2
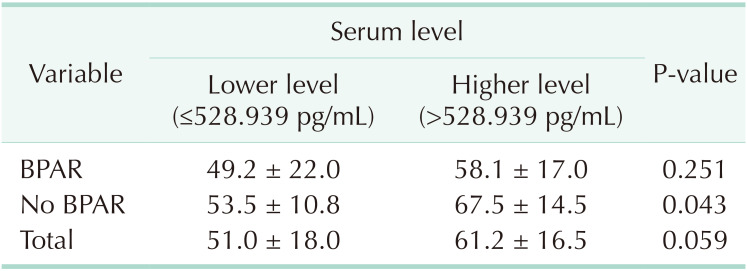
vs. 61.2 ± 16.5 mL/min/1.73 m2, P = 0.059). When subgrouped into patients with or without biopsy-proven acute rejection, 12-month eGFR remained higher in patients receiving kidneys from donors with higher serum Klotho.
Conclusion
Our data demonstrated that donor tissue expression of Klotho correlated with early recovery of eGFR after kidney transplantation. Donor serum Klotho level tended to be associated with posttransplant 12-month eGFR. Donor Klotho expression might be a new predictor for deceased donor kidney transplantation outcome.
Klotho was first identified in 1997 as a novel aging suppressor gene [1]. It encodes a single-pass transmembrane protein containing 3 members, with α-Klotho being the dominant one among them [12]. The kidneys have high protein levels of Klotho and the majority is expressed in distal convoluted tubule cells [3]. There are 2 forms of Klotho protein: a secreted form and a membrane form. The membrane form acts as a coreceptor for fibroblast growth factor-23 (FGF-23), and regulates phosphate absorption and 1,25(OH)2D3 activity [45]. Secreted Klotho protein has multiple functions, including regulation of multiple ion channels and oxidative stress [6].
Klotho is down regulated in acute kidney injury and ischemia reperfusion injury [78]. Hu et al. [6] showed that changes in Klotho level preceded the increase of creatinine or neutrophil gelatinase-associated lipocalin in an animal model of acute kidney injury. In humans, Klotho is known to be decreased in the early stage of chronic kidney disease (CKD) [910]. Some studies demonstrated changes in serum and urine Klotho levels in kidney transplant recipients and donors [1112]. However, research on the association of Klotho with kidney transplantation outcomes is lacking.
This study aimed to measure serum level and renal tissue expression of Klotho in deceased donors and to identify the correlation between Klotho and clinical outcomes of deceased donor kidney transplantation.
Sixty patients who underwent deceased donor kidney transplantation between March 2015 and October 2017 and whose donor blood sample and/or renal tissues were available were enrolled in this study. This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. H-1611-048-807). The study was performed in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants. Blood samples for this study were provided by the Seoul National University Hospital Human Biobank, a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols.
Blood samples of deceased donors were collected prior to organ recovery into a plain tube and stored at −80℃. An 18-G needle core biopsy of the cortex was taken at the time of implantation of the kidney in the recipient.
Klotho expression was identified by immunohistochemistry [14]. Immunohistochemical staining was performed using the Discovery XT automated immunohistochemistry stainer (Ventana Medical Systems, Inc., Tucson, AZ, USA). Formalin-fixed, paraffin-embedded sections (4-mm thickness) were deparaffinized and antigen retrieval was performed using Tris-ethylenediaminetetraacetic acid buffer. Blocking of endogenous peroxides and protein was carried out using a 3% hydrogen peroxide (H2O2) solution. The kidney sections were incubated with 50-µg/mL rat monoclonal anti-Klotho antibody (KM2076; TransGenic Inc., Kobe, Japan) for 32 minutes at 37℃, and subsequently with secondary antibody for 20 minutes at 37℃. Slides were counterstained by hematoxylin and eosin reagent. Nonimmune normal IgG was used to replace primary antibodies as a negative control, and no staining occurred. Digital images were obtained using a Leica SCN400F device. The average percentage of staining tubule cells in 5 randomly selected fields was measured by a pathologist [15].
All of the patients received basilixumab as an induction therapy. Maintenance immunosuppression consisted of steroids, mycophenolate and calcineurin inhibitors.
Graft function was assessed at 1 week and at 1, 3, 6, and 12 months after transplantation based on estimated glomerular filtration rate (eGFR), which was calculated using the Modification of Diet in Renal Disease equation. Delayed graft function (DGF) was defined as the need for hemodialysis within 1 postoperative week. Borderline change and rejection were determined according to the criteria proposed at the 2007 Banff Conference [16].
Protocol biopsy was performed 10 days and 1 year after transplantation. The number of nonsclerotic and globally sclerotic glomeruli was counted to determine the percentage of glomerulosclerosis (GS) [1718]. The severity of interstitial fibrosis and tubular atrophy (IFTA) was graded according to the percentage of cortical area; 1, mild (<25% of cortical area); 2, moderate (25%–50%); 3, severe (>50%) [16].
Continuous data were summarized as the mean ± standard deviation and compared using a t-test and Mann-Whitney test. Categorical data were summarized as proportions and percentages and compared using the chi-square test. Linear regression were used to evaluate the linear relationship between 2 continuous variables. Because of nonnormality, serum Klotho levels were logarithmically transformed. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA).
Blood samples obtained before organ recovery were available in 45 deceased donors. The mean serum level of Klotho was 629.255 ± 354.624 pg/mL (median, 528.939 pg/mL; range, 157.621–1,719.298 pg/mL). The serum Klotho level was not associated with either donor age (r = −0.074, P = 0.622) or donor final creatinine level (r = 0.222, P = 0.148) (Fig. 1).
Renal tissue samples were obtained from 48 patients at the time of transplantation. The mean expression of Klotho in renal tissues was 86.6% ± 10.6% (median, 88.9%; range, 52.1%–99.0%). The mean tissue expression of Klotho gradually decreased in serial kidney biopsies (86.6% ± 10.6% at implantation, 77.9% ± 14.5% at 10-day, and 72.3% ± 14.1% at 1-year posttransplantation) (Fig. 2).
The association of Klotho level and posttransplant GFR were analyzed in 2 ways. Firstly, the patients were divided into 2 groups according to the Klotho serum level of donors and secondly, univariate and multivariate linear regression analysis were used.
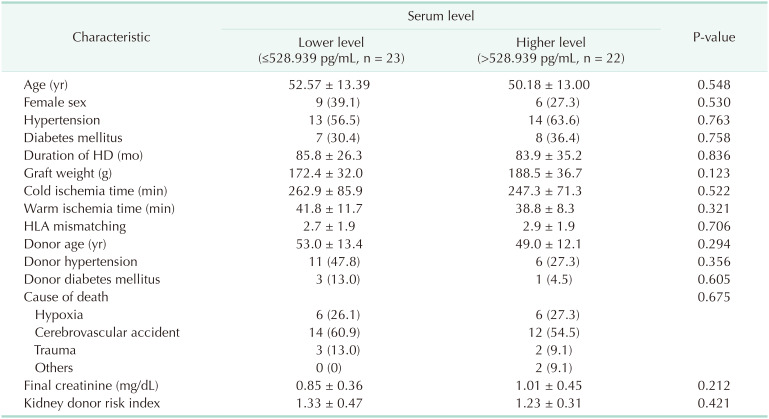
There was no significant difference in the baseline characteristics between the 2 groups divided by the donor Klotho level. The donors with higher Klotho level were younger and showed lower incidence of hypertension and diabetes mellitus, although it did not reach statistical significance (Table 1). There was no significant difference in the incidence of DGF, biopsy-proven acute rejection (BPAR), and borderline changes in both groups (Table 2). The estimated GFR at 12 months after kidney transplantation tended to be higher in patients receiving kidneys from donors with higher serum levels of Klotho (51.0 ± 18.0 mL/min/1.73 m2
vs. 61.2 ± 16.5 mL/min/1.73 m2, P = 0.059).
Patients were subgrouped into 2 groups with or without BPAR to exclude the influence of immunologic insult. Estimated GFR at 12-month remained higher in patients receiving kidneys from donors with higher serum Klotho (Table 3).
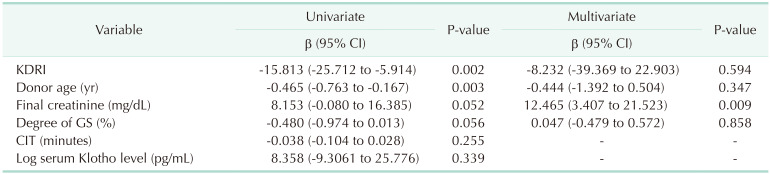
In regression analysis, there was no significant correlation between log transformed donor serum Klotho level and posttransplant eGFR at 12 months. In univariate analysis, kidney donor risk index (KDRI) and donor age were significantly correlated with posttransplant 12 months eGFR. However, they were not confirmed in multivariate analysis. There was a tendency for higher GFR with a higher donor final creatinine level before procurement in both univariate and multivariate analysis (Table 4).
When patients were divided into 2 groups according to the expression of Klotho in the donor renal tissue, there was no statistically significant difference in baseline characteristics (data not shown). There was a tendency for lower incidence of DGF in patients receiving kidneys with higher Klotho expression, although it did not reach statistical significance (Table 2). Patients with kidney grafts with higher Klotho expression showed higher eGFR at 1 week after transplantation (47.5 ± 21.9 mL/min/1.73 m2
vs. 63.9 ± 28.2 mL/min/1.73 m2, P = 0.030). However, the differences were not maintained in the longer-term follow-up of eGFR (Fig. 3).
In multiple regression analysis, there was a significant association between log transformed Klotho expression in renal tissues and eGFR at 1 week after transplantation (β = 0.892, 95% confidence interval, 0.214–1.571; P = 0.011). The analysis included KDRI, donor age, final creatinine in donors, degree of GS in implant biopsy, and cold ischemia time cold ischemia tine, warm ischemia time, and recipient body weight.
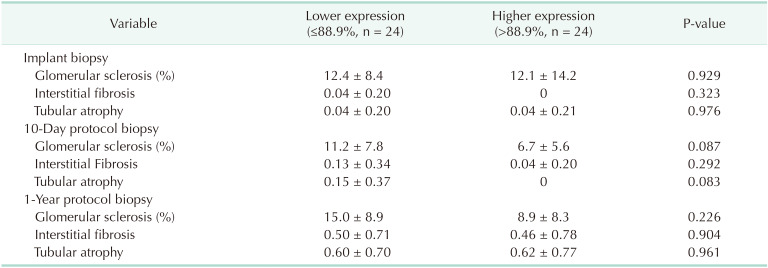
Kidney allografts with higher Klotho expression showed less degree of GS in protocol biopsies taken at 10 days and 1 year after transplantation compared to those with lower Klotho expression, although it did not reach statistical significance (Table 5). There were no significant differences in the degree of IFTA.
In this study, we showed that donor renal tissue expression of Klotho correlated to the recipient eGFR at 1 week after transplantation. Additionally, donor serum Klotho level tended to be associated with recipient eGFR at 1 year after kidney transplantation. Although these results are still preliminary in nature and only showed a tendency, they are meaningful in that it is one of the first attempts to verify a correlation between donor serum and renal tissue expression of Klotho with renal function in kidney transplant recipients. In addition, we performed a biobank study with donor samples obtained all over the county, which showed a role of biobank in transplantation research.
Organ shortage and donor expansion are important issues in kidney transplantation. The proportion of grafts procured from expanded criteria donors has increased, which has led to an increased incidence of DGF and rejection [19]. There is a need for biomarkers to predict the transplant outcomes and to evaluate organ suitability. Serum creatinine level is one of the common criteria for kidney transplantation. However, previous studies have showed favorable outcomes of transplantation using kidneys with acute kidney injury [2021]. Thus, it cannot be an absolute criterion and the decision to use a kidney with rising creatinine frequently depends on physician preference.
Histological findings are sometimes used to evaluate kidneys, although they are not included in the United Network for Organ Sharing criteria or KDRI. GS, IFTA, and hyaline arteriolosclerosis were reported to be associated with poor graft outcomes [222324]. Gaber et al. [25] showed that GS greater than 20% increased DGF and resulted in poor transplant outcomes. However, there have been studies demonstrating limited values of procurement biopsy [2627]. In addition, because there are no defined criteria for which pathological findings can be used to evaluate organ suitability, the decision to discard a kidney varies across surgeons and centers.
Klotho is known to be deficient in early stage of CKD. Both serum and urine Klotho levels were found to be associated with eGFR in patients with CKD [28]. Moreover, Klotho has been previously suggested to be associated with CKD-mineral bone disorder [29]. Contrary to the extensive research on Klotho in CKD, there is limited information on the changes of Klotho after kidney transplantation. Castellano et al. [13] showed down-regulation of Klotho in 7 patients suffering from DGF. A recent article showed that serum Klotho level in donor was lower in older donors and it was negatively associated with short-term kidney outcomes [30].
In the present study, we showed the tendency for increased posttransplant 12-month eGFR in patients receiving kidneys from donors with higher serum Klotho level. While the previous study only showed favorable outcomes at 1 month after transplantation, we followed the patients for a year [30]. Many factors can influence the posttransplant 12-month eGFR. There was no case with recurrence of primary disease or BK virus nephropathy, thus we did subgroup analysis according to the occurrence of BPAR. The posttransplant 12-month eGFR remained higher in the higher donor serum Klotho level group. It means that the positive effect of serum Klotho level on 12-month eGFR remained when the influence of acute rejection was excluded. The association of serum Klotho and posttransplant eGFR was not confirmed in the regression analysis, thus further study with a larger cohort is warranted. In our data, serum Klotho and eGFR showed a trend towards significance as it approached the 12-month period. If the patients were followed up longer, donor Klotho level may be significantly associated with long-term graft function.
Our data showed that there was no correlation between serum Klotho level and donor age or donor final creatinine level, which are widely used parameters for organ suitability. It suggested that Klotho could be a new potential prognostic marker independent of donor age or serum creatinine level. We could not find a correlation between serum level and renal tissue expression of Klotho in donors. Firstly, this might be caused by the small number of patients included in this study. Secondly, the change of protein expression in tissues might be slower than alteration of blood Klotho level. Acute kidney injury could influence the serum level of Klotho especially in deceased donors. Serial measurement and analysis of the correlation between serum and tissue Klotho are warranted to show the concrete results.
There are limitations in this study. First, because it was an observational study with small number of patients, there was a limitation in the statistical analysis. Although the result was promising, further validation with a larger number of patients is required. Second, in terms of methodology, the Klotho ELISA kit is known to give higher readings in fresh serum samples but gave lower readings in the stored samples. Third, both creatinine and Klotho level can be influenced by acute kidney injury caused by ischemic insult in deceased donors. Thus, detailed data on donors, such as length of stay in intensive care unit or episodes of hypotension, could be helpful to analyze the results. Lastly, we could not collect the data on FGF-23 and vitamin D which were closely related to Klotho physiology.
In conclusion, our data demonstrated that renal tissue expression of Klotho was associated with immediate renal function after kidney transplantation. Additionally, donor serum level of Klotho tended to be correlated with posttransplant 12-month eGFR. Donor Klotho expression could be a new predictor for transplant outcomes in deceased donor kidney transplantation. Further studies with larger number of patients are needed to confirm these preliminary results.
ACKNOWLEDGEMENTS
We appreciate Dr. Kang Min Han (Donnguk University College of Medicine) for his histological review of the microscopic slides and Si-Wha Kim for assisting with sample collecting and scanning of slides.
Notes
References
1. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997; 390:45–51. PMID: 9363890.
2. Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013; 33:118–129. PMID: 23465499.
3. Olauson H, Mencke R, Hillebrands JL, Larsson TE. Tissue expression and source of circulating αKlotho. Bone. 2017; 100:19–35. PMID: 28323144.
4. Deng G, Liu D. Klotho: a promising biomarker closely related to kidney transplant. Exp Clin Transplant. 2018; 16:253–258. PMID: 29676702.
5. Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015; 36:174–193. PMID: 25695404.
6. Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010; 78:1240–1251. PMID: 20861825.
7. Izquierdo MC, Perez-Gomez MV, Sanchez-Nino MD, Sanz AB, Ruiz-Andres O, Poveda J, et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol Dial Transplant. 2012; 27 Suppl 4:iv6–iv10. PMID: 23258814.
8. Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol. 2011; 22:1315–1325. PMID: 21719790.
9. Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, et al. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant. 2013; 28:352–359. PMID: 23129826.
10. Barker SL, Pastor J, Carranza D, Quinones H, Griffith C, Goetz R, et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015; 30:223–233. PMID: 25324355.
11. Kimura T, Akimoto T, Watanabe Y, Kurosawa A, Nanmoku K, Muto S, et al. Impact of renal transplantation and nephrectomy on urinary soluble Klotho protein. Transplant Proc. 2015; 47:1697–1699. PMID: 26293036.
12. Leone F, Lofaro D, Gigliotti P, Perri A, Vizza D, Toteda G, et al. Soluble Klotho levels in adult renal transplant recipients are modulated by recombinant human erythropoietin. J Nephrol. 2014; 27:577–585. PMID: 24760622.
13. Castellano G, Intini A, Stasi A, Divella C, Gigante M, Pontrelli P, et al. Complement modulation of anti-aging factor Klotho in ischemia/reperfusion injury and delayed graft function. Am J Transplant. 2016; 16:325–333. PMID: 26280899.
14. Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, et al. α-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015; 100:E1308–E1318. PMID: 26280509.
15. Cho NJ, Han DJ, Lee JH, Jang SH, Kang JS, Gil HW, et al. Soluble Klotho as a marker of renal fibrosis and podocyte injuries in human kidneys. PLoS One. 2018; 13:e0194617. PMID: 29590173.
16. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–760. PMID: 18294345.
17. Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD, et al. Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis. 2016; 68:58–67. PMID: 26857648.
18. Liapis H, Gaut JP, Klein C, Bagnasco S, Kraus E, Farris AB 3rd, et al. Banff histopathological consensus criteria for preimplantation kidney biopsies. Am J Transplant. 2017; 17:140–150. PMID: 27333454.
19. Mundt HM, Yard BA, Kramer BK, Benck U, Schnulle P. Optimized donor management and organ preservation before kidney transplantation. Transpl Int. 2016; 29:974–984. PMID: 26563531.
20. Ugarte R, Kraus E, Montgomery RA, Burdick JF, Ratner L, Haas M, et al. Excellent outcomes after transplantation of deceased donor kidneys with high terminal creatinine and mild pathologic lesions. Transplantation. 2005; 80:794–800. PMID: 16210967.
21. Morgan C, Martin A, Shapiro R, Randhawa PS, Kayler LK. Outcomes after transplantation of deceased-donor kidneys with rising serum creatinine. Am J Transplant. 2007; 7:1288–1292. PMID: 17359500.
22. Karpinski J, Lajoie G, Cattran D, Fenton S, Zaltzman J, Cardella C, et al. Outcome of kidney transplantation from high-risk donors is determined by both structure and function. Transplantation. 1999; 67:1162–1167. PMID: 10232568.
23. Randhawa PS, Minervini MI, Lombardero M, Duquesnoy R, Fung J, Shapiro R, et al. Biopsy of marginal donor kidneys: correlation of histologic findings with graft dysfunction. Transplantation. 2000; 69:1352–1357. PMID: 10798753.
24. Escofet X, Osman H, Griffiths DF, Woydag S, Adam Jurewicz W. The presence of glomerular sclerosis at time zero has a significant impact on function after cadaveric renal transplantation. Transplantation. 2003; 75:344–346. PMID: 12589156.
25. Gaber LW, Moore LW, Alloway RR, Amiri MH, Vera SR, Gaber AO. Glomerulosclerosis as a determinant of posttransplant function of older donor renal allografts. Transplantation. 1995; 60:334–339. PMID: 7652761.
26. Pokorna E, Vitko S, Chadimova M, Schuck O, Ekberg H. Proportion of glomerulosclerosis in procurement wedge renal biopsy cannot alone discriminate for acceptance of marginal donors. Transplantation. 2000; 69:36–43. PMID: 10653377.
27. Wang CJ, Wetmore JB, Crary GS, Kasiske BL. The donor kidney biopsy and its implications in predicting graft outcomes: a systematic review. Am J Transplant. 2015; 15:1903–1914. PMID: 25772854.
28. Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012; 13:155. PMID: 23176706.
29. Zou D, Wu W, He Y, Ma S, Gao J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018; 19:285. PMID: 30348110.
30. Deng G, Yang A, Wu J, Zhou J, Meng S, Zhu C, et al. The value of older donors' Klotho level in predicting recipients' short-term renal function. Med Sci Monit. 2018; 24:7936–7943. PMID: 30396199.
Fig. 1
Correlation between serum Klotho level and final creatinine level before procurement in donor (A) and donor age (B).
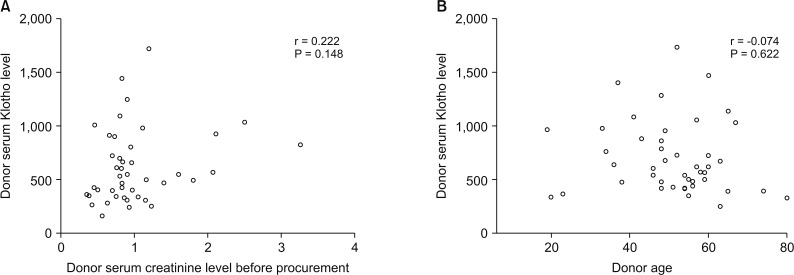
Fig. 2
Immunohistochemistry of an implant (A), a 10-day (B), and a 1-year (C) posttransplantation biopsy (magnification, ×200; scale bar = 100 µm).
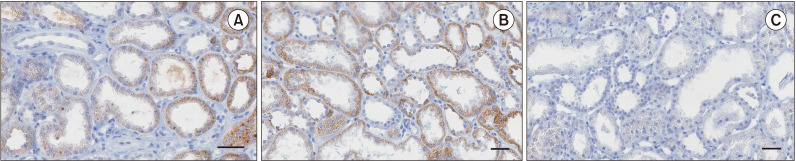
Fig. 3
Estimated glomerular filtration rate (GFR) after transplants according to the expression of Klotho in donor renal tissue.
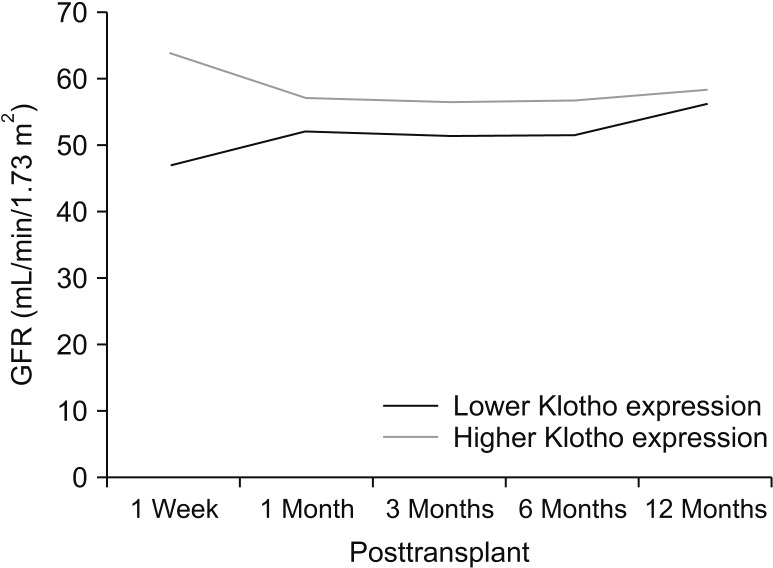
Table 2
Outcomes of kidney transplantation according to donor serum Klotho level and renal tissue Klotho expression
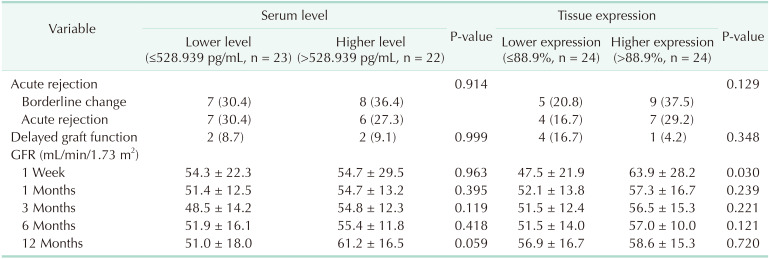
Table 3
Post-transplant 12-month eGFR according to the serum level of Klotho in patients with and without BPAR




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download