Abstract
Purpose
Our previous studies suggested that p53-positive triple-negative breast cancer (TNBC) should be more sensitive to chemotherapy than p53-negative TNBC. The aim of this study was to determine whether p53 expression in TNBC could predict response to neoadjuvant chemotherapy and the resulting prognosis.
Methods
From January 2009 to December 2017, TNBC patients who underwent neoadjuvant chemotherapy were reviewed, including a total of 31 TNBC patients who had clinical lymph node metastasis. The status of p53 expression in patients before and after chemotherapy was evaluated.
Results
Two patients (22.2%, 2 of 9) achieved pCR in p53(+) TNBC and 4 patients (50%, 5 of 10) achieved pCR in p53(−) TNBC. There was no correlation between pCR rate and p53 expression (P = 0.350). Based on prechemotherapy p53 expression, there was no significant difference in disease-free survival (DFS) between p53(+) TNBC and p53(−) TNBC (P = 0.335). However, after chemotherapy, p53(+) TNBC had shown higher DFS than p53(−) TBNC (P = 0.099). Based on prechemotherapy p53 expression, p53(+) TNBC had better overall survival (OS) than p53(−) TNBC, but the difference was not statistically significant (P = 0.082). After chemotherapy, p53(+) TNBC showed significantly better OS than p53(−) TNBC (P = 0.018).
Triple-negative breast cancer (TNBC), which lacks expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), comprises approximately 15% of all invasive breast cancers [1]. TNBC usually has a high histologic grade and exhibits a higher rate of early recurrence and more aggressive behavior compared to other subtypes [12]. Although new targeted therapies such as DNA-damaging poly-adenosine diphosphate ribose polymerase inhibitors and immune-checkpoint inhibitors such as programmed cell death protein 1 and programmed death-ligand 1 have been under investigation in clinical trials, chemotherapy is still used as the main treatment for TNBC.
Despite unfavorable prognoses, TNBCs are known to be highly reactive to chemotherapy and show higher pathologic complete response (pCR) rates after neoadjuvant chemotherapy compared to other types of breast cancer [34], often resulting in a favorable prognosis for patients. However, although pCR is traditionally considered a surrogate marker used to predict the long-term prognosis in TNBC patients [4], TNBC patients with residual cancer have significantly worse prognoses than patients with non-TNBC associated residual cancer [356].
TP53 mutations are the most common genetic alterations in breast cancer and are highly linked to basal subtype carcinomas [78]. Past studies have investigated if TP53 mutation can predict the response of cancer to chemotherapy or affect a difference in prognosis; however, the lack of consistent results has hampered clinical applications. Due to the development of next generation sequencing (NGS), immunostaining has been perceived as a relatively older, less accurate, and less valuable technique for identifying cancer cells. Unfortunately, apart from the cost aspect, limitations remain in interpreting and understanding the clinical implications of NGS results. Because immunostaining studies can be easily performed in most hospitals, those results may be more useful until NGS results are clinically applicable.
We analyzed the prognostic value of immunohistochemically detected p53 in previous studies [910]. In those studies, TNBC patients with p53 expression (p53(+) TNBC) had a worse prognosis than patients without p53 expression (p53(−) TNBC), but chemotherapy significantly increased survival rates [9]. Further, in TNBC patients who received adjuvant chemotherapy, p53 expression was associated with better breast cancer-specific survival rates in patients without lymph node metastasis (stage N0) [10]. Results from these studies suggest that p53(+) TNBC should be more chemosensitive than p53(−) TNBC. The aim of this study was to determine whether p53 expression in TNBC could predict the response to neoadjuvant chemotherapy and the resulting prognosis.
Patients with TNBC who underwent neoadjuvant chemotherapy at Korea University Anam Hospital from January 2009 to December 2017 were reviewed. Thirty-one TNBC patients who exhibited clinical lymph node metastasis were included. Patients with preoperative distant metastasis and those who received adjuvant chemotherapy after surgery were excluded. This study was approved by the Korea University Anam Hospital Institutional Review Board (No. 2018AN0272). Informed consent was not required for this type of study.
Cancers identified as ‘ER-negative,’ ‘PR-negative,’ and ‘HER2-negative’ are defined as ‘triple-negative breast cancer (TNBC).’ When less than 1% of cells exhibit nuclear staining for these receptors by immunohistochemistry (IHC), these cancers are defined as ‘ER-negative’ and ‘PR-negative.’ A ‘HER2-negative’ receptor is defined in the case of 0, +1 or +2 upon IHC or in cases with a HER2/CEP17 ratio <2.0 upon fluorescence in situ hybridization or silver in situ hybridization. Further details of the classification method are described in a previous study [11]. Immunohistochemical evaluation of p53 was performed using a mouse monoclonal anti-human p53 (clone: DO-7) antibody (MA5-12557; DAKO Glostrup, Hovedstaden, Denmark). Staining was performed using an autoimmunostainer (Bond Polymer Refine Detection kit/Leica Bond-Max staining system, Leica Biosystems, Richmond, IL, USA) per the manufacturer's instructions. Nuclear staining >10% was defined as “p53-positive” and staining ≤10% was defined as “p53-negative” [1213]. All diagnoses of p53 were performed by a qualified pathologist (JHL).
Clinical response to neoadjuvant chemotherapy was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 guidelines [14]. Pathologic complete response was defined as the complete absence of invasive cancer in both breast and axillary lymph nodes. Partial response (PR) was defined as a >30% reduction in the largest tumor diameter. Progressive disease (PD) was defined as an increase in tumor size of at least 20% from the base line diameter or the appearance of new disease. Stable disease (SD) was defined as lack of sufficient tumor shrinkage to qualify as PR or sufficient increase in tumor size to qualify as PD.
Differences in the frequencies of clinicopathologic variables were analyzed using Fisher exact test and the chi-squared test (Table 1). Disease-free survival (DFS) was defined as the time from surgery to the date of documentation of relapse, including locoregional recurrence and/or distant metastasis. Overall survival (OS) was defined as the time from surgery to the date of death. Survival curves were constructed using the Kaplan-Meier method. Statistical significance was defined as P < 0.05. All statistical analyses were performed using IBM SPSS Statistics 20.0 (IBM Corp., New York, NY, USA).
It was possible to interpret p53 expression in both specimens from 18 patients before and after chemotherapy. Excluding 6 patients that achieved pCR, 83.3% (10 of 12) of patients did not experience change in p53 expression, which included 6 patients with p53(+) TNBC and 4 patients with p53(−) TNBC. Two patients had a change from p53(−) TNBC to p53(+) TNBC.
Two patients (22.2%, 2 of 9) achieved pCR in p53(+) TNBC and 4 patients (50%, 5 of 10) achieved pCR in p53(−) TNBC. There was no correlation between pCR rate and p53 expression. (P = 0.350). There was no SD in p53(−) TNBC. There were no significant differences in DFS (P = 0.438) or OS (P = 0.715) related to pCR (Fig. 1).
Based on prechemotherapy p53 expression, there was no significant difference in DFS between p53(+) TNBC and p53(−) TNBC (P = 0.335). However, after chemotherapy, p53(+) TNBC showed higher DFS than p53(−) TBNC (P = 0.099) (Fig. 2).
Based on prechemotherapy p53 expression, p53(+) TNBC had better OS than p53(−) TNBC, but the difference was not statistically significant (P = 0.082). After chemotherapy, p53(+) TNBC showed significantly better OS than p53(−) TNBC (P = 0.018).
There were no significant differences in prognosis by clinical T stage (DFS, P = 0.680; OS, P = 0.878) or pathologic T stage (DFS, P = 0.174; OS, P = 0.834). By clinical N stage, there was no significant difference in DFS (P = 0.366), but there was a significant difference in OS (P = 0.014) (Fig. 3); alternatively, there was a significant difference in DFS (P = 0.023) but no significant difference in OS (P = 0.291) by pathologic N stage (yp Nstage) (Fig. 3).
Including all patients, 22 (70.9%) received 6 cycles of docetaxel + epirubicin (TE) chemotherapy. There was no difference in survival by chemotherapy regimen. In those patients who received TE chemotherapy, p53(+) TNBC had a higher OS than p53(−) TNBC, but the difference was not statistically significant. There was no difference in DFS between p53(+) TNBC and p53(−) TNBC.
We reported the clinical significance of immunohistochemically detected p53 in previous studies. In a study of TNBC patients receiving adjuvant chemotherapy in a single center, p53(+) TNBC showed a better prognosis than p53(−) TNBC in early stages without lymph node metastasis [10]. Analysis of the Korean Breast Cancer Society Registry database revealed that p53(+) TNBC had a poorer prognosis than p53(−) TNBC in patients who did not receive chemotherapy, but there was no difference in survival rates between the 2 groups after receiving adjuvant chemotherapy. Chemotherapy was associated with a decrease in the hazard ratio (HR) in p53(+) TNBC, but there was no chemotherapy-associated reduction of HR in p53(−) TNBC [9]. This suggested that p53(+) TNBC was more sensitive to chemotherapy than p53(−) TNBC. Therefore, we investigated if there was any difference in chemotherapy response according to p53 expression in patients undergoing neoadjuvant chemotherapy.
This study has limitations in that the sample size was relatively small; however, it is important to gain insight and suggest the need for further research. First, we examined whether there was a difference in chemotherapy response according to p53 expression. Both pCR and PR were lower in P53(+) TNBC than in p53(−) TNBC. According to the RECIST evaluation criteria, p53(−) TNBC showed better response that p53(+) but there was no significant difference between the 2 groups. In this study, pCR was not predicted by p53 expression, and there was no difference between pCR patients and non-pCR patients.
Previous studies have suggested that pCR is a surrogate marker of a good prognosis. Results from a phase III trial (EORTC 10994/BIG 1-00) showed that pCR was a predictor of a good prognosis in all subtypes [15]. However, results vary as to whether TP53 mutation can predict the response to chemotherapy. Wang et al. [16] showed that the significance of TP53 mutation differed depending on the type of chemotherapy, as TP53 mutations were more likely to respond to anthracycline/cyclophosphamide-based neoadjuvant chemotherapy which would result in higher survival. Among patients treated with paclitaxel, there was no significant difference in pCR rates by TP53 mutations. In the neoadjuvant GeparSixto trial, despite the high incidence of TP53 mutations in TNBC with HER2-positive breast cancer, TP53 mutation did not predict the response to neoadjuvant chemotherapy in these subtypes [17]. In an NGS study, P53BP2 amplifications were associated with a poor response [8]. Knappskog et al. [18] showed that “concomitant inactivation of the p53- and pRB-pathways predict resistance towards anthracyclines and mitomycin in breast cancer in vivo.”
Resultant differences in previous studies may be due to the heterogeneity of the study subjects. Moreover, past studies used different types and doses of chemotherapy and used different evaluation criteria for p53 expression. Lee et al. [19] showed that p53 in HR+ and HR- breast cancer was not a predictor of response to neoadjuvant chemotherapy in immunohistochemical studies, where more than 50% of the p53 gene expression was positive and patients were treated with 3 to 7 cycles of docetaxel (75 mg/m2) and doxorubicin (50 mg/m2). Kim et al. [20] showed a significant association with p53 expression and pCR after neoadjuvant chemotherapy in TNBC patients. In this study, more than 50% of the p53 expression was positive, and patients were treated with 3 to 6 cycles of docetaxel and doxorubicin, and 4 cycles of AC followed by 4 cycles of docetaxel.
Second, p53 expression was compared before and after chemotherapy. There was no change in p53(+) TNBC before chemotherapy to p53(−) TNBC after chemotherapy. Lee et al. [19] reported that the expression of PR and Ki67 were reduced after neoadjuvant chemotherapy. 86.7% (26 of 30) of patients did not show a change in p53 status after chemotherapy (p53(+), 33 patients; p53(−), 13 patients) and only 13.7% (4 of 30) showed this change after neoadjuvant chemotherapy. These rates were similar in a TP53 mutation study: changes included WT → WT (171 of 206, 83.0%), MT → WT (21 of 206, 10.2%), and WT → MT (7 of 206, 3.4%), and there were no differences in DFS [21].
Finally, in this study, the most compelling result was that p53(+) TNBC had a better prognosis than p53(−) TNBC in patients who underwent neoadjuvant chemotherapy. p53(+) TNBC, regardless of pCR, had better OS than p53(−) TNBC. No patient died during the follow-up period for p53(+) TNBC. In p53(−) TNBC patients, 1 out of 4 pCR patients and 2 out of 6 non-pCR patients died. Previous studies have shown that chemotherapy improved p53(+) TNBC prognosis and that p53(+) TNBC is more prognostic than p53(−) TNBC in early TNBC treated with adjuvant chemotherapy [9]. As previously discussed, the p53 protein does not accurately reflect TP53, but wild-type p53 is mostly a mutant type because of its short half-life. ER-negative-breast cancers with the TP53 mutation and resultant genetic abnormalities would lead to TP53-independent apoptosis and mitotic catastrophe and would better respond to chemotherapy than wild-type p53 [2223].
Recent studies indicated that resistance to chemotherapy is likely when TP53 mutation and other defects occur together. Patients with initial TP53 and PIK3CA mutations showed a better prognosis than those with no mutations if there was a change to negative mutation after neoadjuvant chemotherapy [21]. “TP53 and CHEK2 mutations were associated with lack of response to epirubicin monotherapy. In contrast, TP53 mutations and MDM2 309G allele status conferred poor disease-specific survival among patients treated with primary paclitaxel but not epirubicin monotherapy” [24]. In locally advanced breast cancer research, TP53 mutations were predictive of resistance to doxorubicin. TP53 mutations that affect the L2 or L3 DNA binding domain of the p53 protein were associated with lack of response to anthrathyclins [25].
In conclusion, immunohistochemically detected p53 expression in TNBC did not predict the response to neoadjuvant chemotherapy. However, patients with p53(+) TNBC had better OS than patients with p53(−) TNBC who underwent neoadjuvant chemotherapy. In this regard, further research on chemotherapy drugs is needed.
Notes
References
1. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010; 363:1938–1948. PMID: 21067385.
2. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13:4429–4434. PMID: 17671126.
3. Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26:1275–1281. PMID: 18250347.
4. von Minckwitz G, Martin M. Neoadjuvant treatments for triple-negative breast cancer (TNBC). Ann Oncol. 2012; 23 Suppl 6:vi35–vi39. PMID: 23012300.
5. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804. PMID: 22508812.
6. National Comprehensive Cancer Network. NCCN Guidelines Version 3. 2018 Breast Cancer [Internet]. Fort Wathington (PA): National Comprehensive Cancer Network;c2018. cited 2018 Dec 3. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
7. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012; 490:61–70. PMID: 23000897.
8. Lips EH, Michaut M, Hoogstraat M, Mulder L, Besselink NJ, Koudijs MJ, et al. Next generation sequencing of triple negative breast cancer to find predictors for chemotherapy response. Breast Cancer Res. 2015; 17:134. PMID: 26433948.
9. Bae SY, Nam SJ, Jung Y, Lee SB, Park BW, Lim W, et al. Differences in prognosis and efficacy of chemotherapy by p53 expression in triple-negative breast cancer. Breast Cancer Res Treat. 2018; 172:437–444. PMID: 30132220.
10. Bae SY, Jung SP, Lee SK, Yu J, Lee JE, Kim SW, et al. Prognostic value of immunohistochemically detected p53 in adjuvant chemotherapy-treated triple negative breast cancer. Kaohsiung J Med Sci. 2018; 34:663–672. PMID: 30527200.
11. Cho DH, Bae SY, You JY, Kim HK, Chang YW, Choi YJ, et al. Lymph node ratio as an alternative to pN staging for predicting prognosis after neoadjuvant chemotherapy in breast cancer. Kaohsiung J Med Sci. 2018; 34:341–347. PMID: 29747778.
12. Fountzilas G, Giannoulatou E, Alexopoulou Z, Zagouri F, Timotheadou E, Papadopoulou K, et al. TP53 mutations and protein immunopositivity may predict for poor outcome but also for trastuzumab benefit in patients with early breast cancer treated in the adjuvant setting. Oncotarget. 2016; 7:32731–32753. PMID: 27129168.
13. Lara JF, Thor AD, Dressler LG, Broadwater G, Bleiweiss IJ, Edgerton S, et al. p53 Expression in node-positive breast cancer patients: results from the Cancer and Leukemia Group B 9344 Trial (159905). Clin Cancer Res. 2011; 17:5170–5178. PMID: 21693655.
14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.
15. Bonnefoi H, Litiere S, Piccart M, MacGrogan G, Fumoleau P, Brain E, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol. 2014; 25:1128–1136. PMID: 24618153.
16. Wang Y, Xu Y, Chen J, Ouyang T, Li J, Wang T, et al. TP53 mutations are associated with higher rates of pathologic complete response to anthracycline/cyclophosphamide-based neoadjuvant chemotherapy in operable primary breast cancer. Int J Cancer. 2016; 138:489–496. PMID: 26238069.
17. Darb-Esfahani S, Denkert C, Stenzinger A, Salat C, Sinn B, Schem C, et al. Role of TP53 mutations in triple negative and HER2-positive breast cancer treated with neoadjuvant anthracycline/taxane-based chemotherapy. Oncotarget. 2016; 7:67686–67698. PMID: 27611952.
18. Knappskog S, Berge EO, Chrisanthar R, Geisler S, Staalesen V, Leirvaag B, et al. Concomitant inactivation of the p53- and pRB- functional pathways predicts resistance to DNA damaging drugs in breast cancer in vivo. Mol Oncol. 2015; 9:1553–1564. PMID: 26004085.
19. Lee HC, Ko H, Seol H, Noh DY, Han W, Kim TY, et al. Expression of immunohistochemical markers before and after neoadjuvant chemotherapy in breast carcinoma, and their use as predictors of response. J Breast Cancer. 2013; 16:395–403. PMID: 24454461.
20. Kim T, Han W, Kim MK, Lee JW, Kim J, Ahn SK, et al. Predictive significance of p53, Ki-67, and Bcl-2 expression for pathologic complete response after neoadjuvant chemotherapy for triple-negative breast cancer. J Breast Cancer. 2015; 18:16–21. PMID: 25834606.
21. Jiang YZ, Yu KD, Bao J, Peng WT, Shao ZM. Favorable prognostic impact in loss of TP53 and PIK3CA mutations after neoadjuvant chemotherapy in breast cancer. Cancer Res. 2014; 74:3399–3407. PMID: 24924774.
22. Varna M, Lehmann-Che J, Turpin E, Marangoni E, El-Bouchtaoui M, Jeanne M, et al. p53 dependent cell-cycle arrest triggered by chemotherapy in xenografted breast tumors. Int J Cancer. 2009; 124:991–997. PMID: 19048622.
23. Jackson JG, Pant V, Li Q, Chang LL, Quintas-Cardama A, Garza D, et al. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell. 2012; 21:793–806. PMID: 22698404.
24. Chrisanthar R, Knappskog S, Lokkevik E, Anker G, Ostenstad B, Lundgren S, et al. Predictive and prognostic impact of TP53 mutations and MDM2 promoter genotype in primary breast cancer patients treated with epirubicin or paclitaxel. PLoS One. 2011; 6:e19249. PMID: 21556366.
25. Geisler S, Lonning PE, Aas T, Johnsen H, Fluge O, Haugen DF, et al. Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer. Cancer Res. 2001; 61:2505–2512. PMID: 11289122.
Fig. 1
Survival plotted by chemotherapy response: (A) disease-free survival and (B) overall survival. pCR, pathologic complete response.
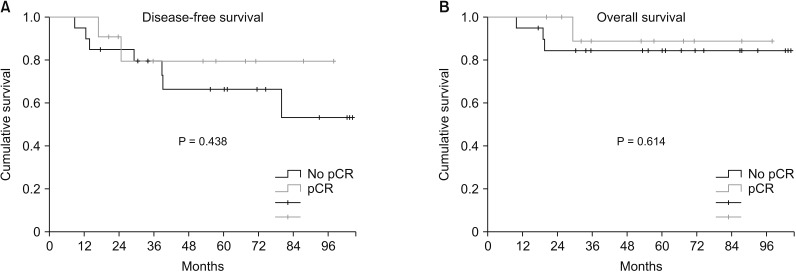
Fig. 2
Survival plotted by p53 expression: (A) disease-free survival (DFS) by prechemotherapy p53 status, (B) disease-free survival by postchemotherapy p53 status, (C) overall survival by prehemotherapy p53 status, and (D) overall survival by posthemotherapy p53 status.
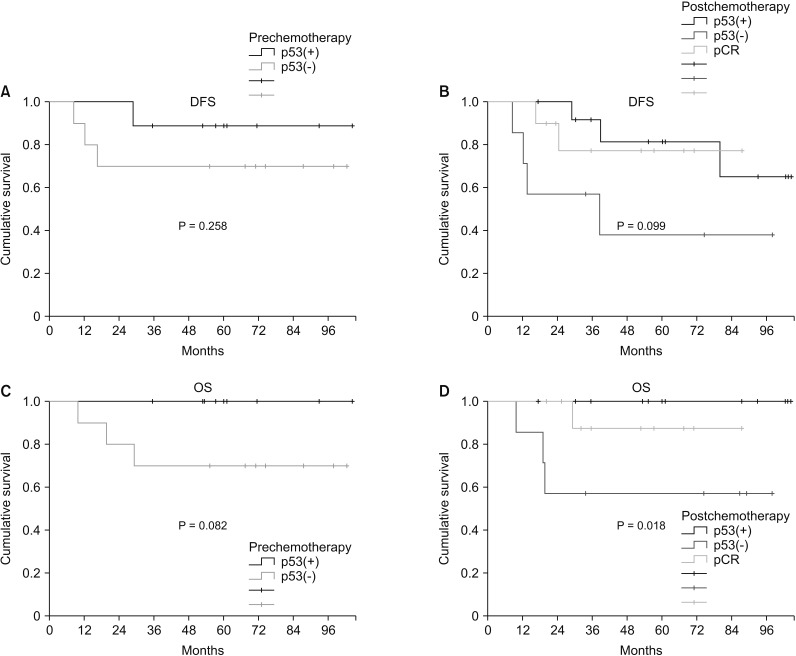
Fig. 3
Survival plotted by N stage; (A) disease-free survival by clinical N stage, (B) overall survival by clinical N stage, (C) disease-free survival by pathologic N stage (yp N stage), and (D) overall survival by pathologic N stage.
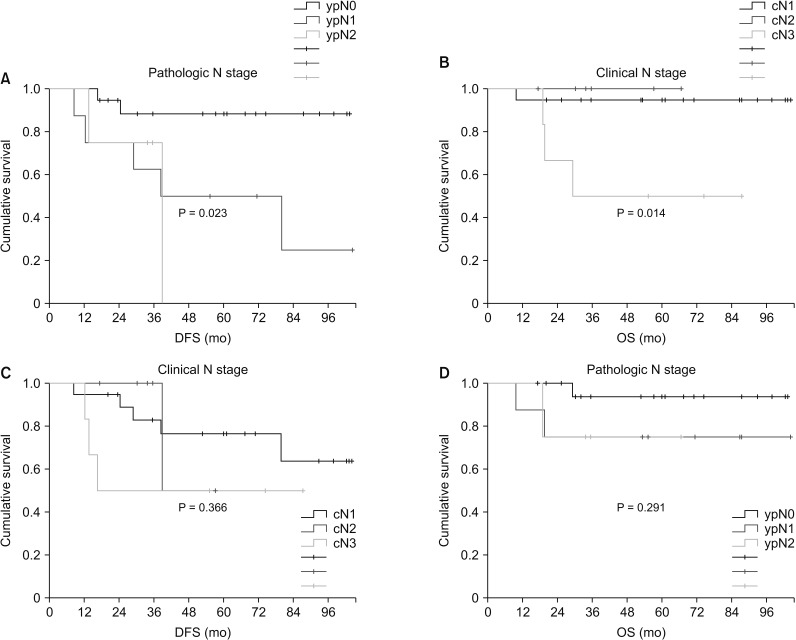
Table 1
The clinicopathologic characteristics of the total patients

Values are presented as number (%) unless otherwise indicated.
BCS, breast conserving surgery; MRM, modified radical mastectomy; IDC, invasive ductal carcinoma; pCR, pathologic complete response; pCR, pathologic complete response; AC, doxorubicin/cyclophosphamide; AC-T, AC followed by docetaxel; TE, docetaxel and epirubicine.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download