Abstract
Purpose
We evaluated the changes in body composition (BC) and quality of life (QoL) in patients who underwent gastrectomy for advanced gastric cancer.
Methods
BC data using segmental multifrequency bioelectrical impedance analysis and QoL data from the EORTC (European Organisation for the Research and Treatment of Cancer) gathered via QLQ-C30 and QLQ-STO22 questionnaires were obtained from 300 patients preoperatively and at 1, 2, and 3 years after surgery. In total, 114 patients underwent total gastrectomy (TG group) and 186 underwent distal gastrectomy (DG group).
Results
According to BC analysis, at 3 years postoperatively, the average body weight (P = 0.002), protein mass (P = 0.028), body fat mass (P = 0.009), skeletal muscle mass (P = 0.037), and visceral fat area (P = 0.012) was significantly decreased in the TG group than in the DG group compared to the preoperative. In the QLQ-C30, physical functioning (P = 0.001), role functioning (P = 0.013), and fatigue (P = 0.005) showed significantly worse QoL in the TG group than in the DG group at 2 and 3 years postoperatively. In the QLQ-STO22, pain (P = 0.001), reflux symptoms (P = 0.009), eating restrictions (P = 0.001), anxiety (P = 0.008), taste (P = 0.011), and body image (P = 0.014) showed greater continuous deterioration postoperatively in the TG group than in the DG group.
Despite progress in cancer treatment, surgical resection is the primary treatment option for advanced gastric cancer. Unlike early stages of gastric cancer, in which function preserving gastrectomy can be performed, distal subtotal gastrectomy or total gastrectomy (TG) is the standard treatment for advanced gastric cancer [1]. However, postgastrectomy symptoms due to surgical resection, which result in loss of reservoir capacity, are inevitable. These postgastrectomy symptoms lead to deterioration in patient quality of life (QoL) and body composition (BC) status and can vary depending on the extent of the gastrectomy [2].
Patient survival is always the highest priority in the treatment of cancer. However, focusing on patient survival takes away from preserving QoL. Recently, many studies on the QoL after gastrectomy have been published. Most of the previous studies compared QoL changes to the extent of surgical treatment or the impact of time throughout the postoperative survival period [345]. However, studies that evaluate changes in QoL associated with different types of surgical procedures in patients with advanced gastric cancer who have factors that can significantly affect QoL, such as tumor burden and adjuvant chemotherapy, are important.
Alterations in BC after gastrectomy are a common phenomenon. In particular, body weight loss has been recognized as an unavoidable complication after gastrectomy and these changes can affect postoperative nourishment and QoL in the patient [6]. However, there are currently few reports depicting the long-term changes in BC after gastrectomy and most of the previous studies focused on immediate postoperative BC changes [78]. Long-term BC change data, including the time until the end of adjuvant chemotherapy, may provide information for appropriate medical intervention in patients with advanced gastric cancer.
To explore optimal time points for medical interventions in patients who underwent gastrectomy for advanced gastric cancer, we conducted a prospective observational study to compare QoL and BC status between the distal gastrectomy (DG) and TG for 3 years after surgery.
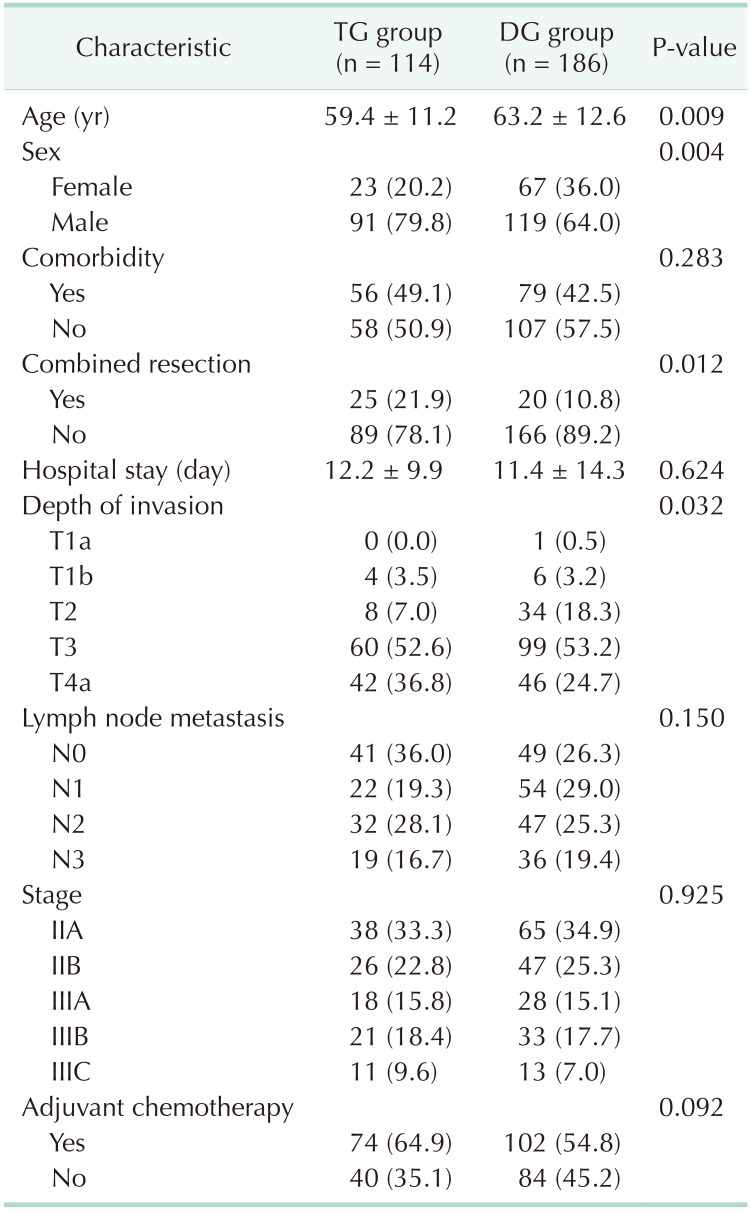
Patients with pathological stages II and III gastric cancer who underwent curative gastrectomy between January 2011 and June 2014 at the Kyungpook National University Chilgok Hospital were enrolled. We excluded patients who experienced a recurrence during the follow-up period as well as those who expired of other diseases. In total, 114 patients were in the TG group and 186 patients were in the DG group. The patients completed the entire series of BC and QoL assessments during the 3-year following surgery. The Institutional Review Board (IRB) of Kyungpook National University Chilgok Hospital approved this study (approval numbers: 2019-01-018). Written informed consent was waived by the IRB.
In the TG, Roux-en-Y esophagojejunostomy was performed extracorporeally using circular staplers. In the DG, Billroth I anastomosis using a circular stapler, or Billroth II anastomosis was performed. Curative gastrectomy with D2 lymph node dissection, including total omentectomy was performed in all patients [9]. After surgery, patients were placed on a diet program that included drinking water on the third postoperative day, followed by a liquid and soft diet. Patients were planned to be discharged on the sixth day, postoperatively.
BC was examined by segmental multifrequency bioelectrical impedance analysis using InBody 720 (Biospace, Seoul, Korea) preoperatively and annually up to 3 years after surgery. According to the manual provided by the manufacturer, the patients were to be examined in the morning before meals and exercise, if possible. The patients stood on the foot electrodes in a relaxed upright position while loosely gripping the hand electrodes. The impedance method measures each item of BC through body resistance. The body mass index (BMI) was calculated as body weight/height2 (kg/m2), and the degree of obesity was calculated as body weight/ideal body weight (%).
The European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and EORTC QLQ-STO22 questionnaires were translated into a validated Korean Version and used to assess the QoL in patients [10]. Patients were asked to complete the QLQ-C30 and QLQ-STO22 questionnaires 4 times: preoperatively, and at 1, 2, and 3 years following surgery. The preoperative QoL assessment was performed when patients were hospitalized for surgery; alternatively, the postoperative QoL assessment was performed at the outpatient department. The raw scores were linearly transformed into scores ranging from 0 to 100, according to the manual provided by the EORTC. QoL was based on the preoperative score and analyzed as the amount of change at each time point after surgery. For global health status/QoL and functional scales, an increased QoL score postoperatively could be interpreted as high QoL and improved functioning after surgery. However, in symptom scales and QLQ-STO22, this pattern reflects more symptoms/problems after surgery.
Differences in baseline characteristics of patients between the 2 groups were analyzed using Student t-test for continuous variables and the chi-square test for categorical variables.
Linear mixed models were used to assess how the surgery affected the changes between the groups and over time. If there was a significant difference between the 2 groups, we analyzed the time point at which the 2 groups differed. The P-value, corrected by Bonferroni post hoc testing, was calculated for the comparison of differences in mean scores among the time intervals. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA).
Table 1 shows the clinicopathological characteristics of the TG and DG groups. There were no statistical differences in length of hospital stay, adjuvant chemotherapy, comorbidity, lymph node metastasis, and pathological stage between the TG and DG groups. However, age, sex, combined resection, and depth of invasion showed statistical differences between the 2 groups.
We compared the mean BC between the groups and over time (Fig. 1). According to BC analysis, at 3 years postoperatively, average body weight (P = 0.002), protein mass (P = 0.028), body fat mass (P = 0.009), skeletal muscle mass (P = 0.037), and visceral fat area (P = 0.012) were significantly lower in the TG group than in the DG group compared to the preoperative status. Change in body weight was significantly lower in the TG group than in the DG group at all time points after surgery. Protein mass and skeletal muscle mass showed continuous decreases in both groups after surgery, but the rate of change was more significant at 3-year postsurgery compared to preoperatively. Body fat mass and visceral fat area were lowest 1 year after surgery in both groups but recovered in the following years. During the second and third years after surgery, the points at which body fat mass and visceral fat area showed recovery, the rate of change was significantly less in the DG group than the TG group compared to preoperative rates. Fat-free mass and edema index showed similar patterns in both groups after surgery, without a significant difference.
We compared the mean QoL score between the groups and over time. In the QLQ-C30 assessment, physical functioning (P = 0.001), role functioning (P = 0.013), and fatigue (P = 0.005) showed significantly worse QoL in the TG group than in the DG group at 2 and 3 years postoperatively (Figs. 2, 3). Global health status showed improvement after surgery in both groups, but no significant differences were noted. In functional scales, the 2 groups showed different aspects of change in physical functioning and role functioning. The difference was significant at 2 and 3 years after surgery and the DG group showed better QoL than the TG group. In symptom scales, fatigue was worse in the TG group than in the DG group after surgery and the difference was significant at 2 and 3 years after surgery. Financial difficulties showed significant differences between the 2 groups and over time; however, there was no difference between the preoperative and postoperative periods. In the QLQ-STO22 assessment, pain (P = 0.001), reflux symptoms (P = 0.009), eating restrictions (P = 0.001), anxiety (P = 0.008), taste (P = 0.011), and body image (P = 0.014) showed greater continuous deterioration postoperatively in the TG group than in the DG group (Fig. 4). The DG group showed better QoL after surgery compared to the TG group in all items, but the time point at which significant differences were found varied for each item. As shown in Fig. 4, pain, reflux symptoms, eating restrictions, and anxiety were different at all time points after surgery. The difference in taste occurred at one and 3 years after surgery and differences in body image occurred at 2 and 3 years after surgery.
Concerning BC changes in our study, the TG group showed a significant change compared to the DG group. The most significant change was observed at 1-year postsurgery, and subsequent changes in BC items differed in the following years. BC changes that occurred 3 years after surgery can be divided into 2 groups. First, it can include an item such as body weight, body fat mass, BMI, and visceral fat area that decreased significantly during the first year after surgery and maintained a decreased level or was slightly increased during the next 2 years after surgery. These BC items indicate that the patient will be stabilized 1 year after the surgery. Patients should also be informed that these BC items will remain at a reduced state until 3 years following surgery. Second, it can include an item such as protein mass, fat-free mass, and skeletal muscle mass that continued to decrease for 3 years after surgery. These BC items require appropriate medical intervention such as rehabilitation training and nutrition counseling for patients during the follow-up period. As in our study, changes in these 2 categories were significantly greater in patients who underwent TG; thus, intensive follow-up and active medical intervention are needed.
There are many studies on QoL changes after gastrectomy and it is well known that QoL after TG results in a worse QoL than DG [5111213]. Our study aimed to determine the differences in QoL between the 2 groups at certain time points after surgery and to investigate the significance of these differences at each time point. There are some items in the QoL questionnaire that show a significant difference between the 2 groups from the first year after surgery, and there are some items that change from the second year after surgery. These results may indicate that supportive care should be initiated at different time points to improve QoL after surgery. In our study, EORTC STO22 items such as pain, reflux, eating restriction, and anxiety showed more deterioration in QoL in the TG group than in the DG group 1 year after surgery. These differences in QoL may be affected by various factors; however, QoL items that differ from the first year after surgery may be influenced by the surgical procedure itself. These QoL items require sustained supportive care and medical intervention after surgery. For example, administering medication to improve pain, reflux, and eating restriction may improve QoL. The differences in QoL items related to anxiety need to be understood from a different point of view. It is difficult to say that simply performing TG affects anxiety. To understand the high anxiety score associated with TG, anxiety questionnaire items should be revised. The EORTC QLQ-STO22 anxiety questionnaire included the query “Have you worried about your weight being too low?”. The questionnaire composition of the anxiety item includes items related to weight loss. The anxiety questionnaire simply includes the patient's worry about a recurrence of cancer or general health status, but also the fear of weight loss. Therefore, it is helpful to analyze BC changes to identify anxiety items among QoL items. To reduce the anxiety of patients who underwent TG, it is necessary to inform the patients about the reduction in weight far in advance through the BC analysis and to eliminate worries about weight changes.
Patients diagnosed with gastric cancer are increasingly interested in what medical intervention should be done after surgery. Among them, understanding the changes in QoL and BC is an important part of medical intervention that can be provided to patients after surgery. However, some surgeons believe that patients need to endure and tolerate the inevitable QoL and BC changes caused by the altered gastrointestinal tract following surgery. Many symptoms that occur in patients after surgery may sometimes be ignored. To investigate these symptoms objectively, the QoL questionnaire or BC analysis is performed. Many studies have been conducted on the QoL and altered BC of patients after gastrectomy. However, most studies focus on the QoL or BC that has changed after surgery rather than investigating the medical interventions that can be administered to the patient [141516]. Also, in practice, it is not an easy process to significantly improve QoL and BC changes during postoperative follow-up. In our study, we confirmed the QoL and BC changes according to the overall flow. Furthermore, we confirmed whether there was a difference in the amount of change between the 2 groups at different time points. The significance of this study can be attributed to the differences in the QoL and BC between the groups at specific time points after surgery. Further studies are needed to determine the optimal time point for medical intervention after gastrectomy.
The QoL questionnaire can be influenced by various factors and can be regarded as a relative and subjective concept due to various characteristics inherent to each patient [1718]. In our study, we analyzed the QoL and the changes in BC in patients who underwent TG or DG by performing the BC assessment and assumed that decreases in body weight negatively affected the emotional aspect of patient QoL. The QoL questionnaire items were affected by various factors; thus, it was challenging to identify causal relationships. Therefore, QoL questionnaire items may be unreasonable as an absolute QoL evaluation tool, and more comprehensive evaluation is required that incorporates other analyses such as BC.
In conclusion, persistent deterioration of BC and QoL changes are a major concern after TG. The ideal medical postoperative intervention needed for such patients can be identified by assessing BC and QoL. Long-term management of BC is required after gastrectomy and efforts should be made to improve the QoL of patients as soon as possible, postoperatively.
References
1. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.
2. Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, et al. Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg. 2012; 256:1008–1013. PMID: 23154395.
3. Yu W, Park KB, Chung HY, Kwon OK, Lee SS. Chronological changes of quality of life in long-term survivors after gastrectomy for gastric cancer. Cancer Res Treat. 2016; 48:1030–1036. PMID: 27004956.

4. Park KB, Lee SS, Kwon OK, Chung HY, Yu W. Chronological changes in quality of life after distal gastrectomy for gastric cancer. J Gastric Cancer. 2017; 17:110–119. PMID: 28680716.

5. Lee SS, Chung HY, Kwon OK, Yu W. Long-term quality of life after distal subtotal and total gastrectomy: symptom- and behavior-oriented consequences. Ann Surg. 2016; 263:738–744. PMID: 26501699.
6. Katsube T, Konnno S, Murayama M, Kuhara K, Sagawa M, Yoshimatsu K, et al. Changes of nutritional status after distal gastrectomy in patients with gastric cancer. Hepatogastroenterology. 2008; 55:1864–1867. PMID: 19102410.
7. Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, et al. Postoperative changes in body composition after gast rectomy. J Gast rointest Surg. 2005; 9:313–319.
8. Aoyama T, Sato T, Hayashi T, Yamada T, Cho H, Ogata T, et al. Does a laparoscopic approach attenuate the body weight loss and lean body mass loss observed in open distal gastrectomy for gastric cancer? A single-institution exploratory analysis of the JCOG 0912 phase III trial. Gastric Cancer. 2018; 21:345–352. PMID: 28623524.

9. Guideline Committee of the Korean Gastric Cancer Association (KGCA). Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019; 19:1–48. PMID: 30944757.
10. Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004; 13:863–868. PMID: 15129896.

11. Park S, Chung HY, Lee SS, Kwon O, Yu W. Serial comparisons of quality of life after distal subtotal or total gastrectomy: what are the rational approaches for quality of life management? J Gastric Cancer. 2014; 14:32–38. PMID: 24765535.

12. Karanicolas PJ, Graham D, Gonen M, Strong VE, Brennan MF, Coit DG. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg. 2013; 257:1039–1046. PMID: 23665970.
13. Lee JH, Lee HJ, Choi YS, Kim TH, Huh YJ, Suh YS, et al. Postoperative quality of life after total gastrectomy compared with partial gastrectomy: longitudinal evaluation by European Organization for Research and Treatment of Cancer-OG25 and STO22. J Gastric Cancer. 2016; 16:230–239. PMID: 28053809.

14. Park KB, Kwon OK, Yu W. Midterm body composition changes after open distal gastrectomy for early gastric cancer. Ann Surg Treat Res. 2018; 95:192–200. PMID: 30310802.

15. Park KB, Park JY, Lee SS, Kwon OK, Chung HY, Yu W. Impact of body mass index on the quality of life after total gastrectomy for gastric cancer. Cancer Res Treat. 2018; 50:852–860. PMID: 28903552.

16. Park KB, Yu B, Park JY, Kwon OK, Yu W. Impact of body mass index on quality of life after distal gastrectomy for gastric cancer. Ann Surg Treat Res. 2019; 96:250–258. PMID: 31073515.

17. Kim JW, Kim JG, Kang BW, Chung IJ, Hong YS, Kim TY, et al. Treatment patterns and changes in quality of life during first-line palliative chemotherapy in Korean patients with advanced gastric cancer. Cancer Res Treat. 2019; 51:223–239. PMID: 30584995.

18. Tanaka C, Kanda M, Murotani K, Yoshikawa T, Cho H, Ito Y, et al. Long-term quality of life and nutrition status of the aboral pouch reconstruction after total gastrectomy for gastric cancer: a prospective multicenter observational study (CCOG1505). Gastric Cancer. 2019; 22:607–616. PMID: 30382468.

Fig. 1
Chronological changes in body composition. TG, total gastrectomy; DG, distal gastrectomy. *Significant changes between the groups and over time.

Fig. 2
Chronological changes in quality of life (global health status and functional scales of EORTC QLQ-C30). EORTC, European Organisation for the Research and Treatment of Cancer; GH, global health status; PF2, physical functioning; RF2, role functioning; EF, emotional functioning; CF, cognitive functioning; SF, social functioning. *Significant changes between the groups and over time.
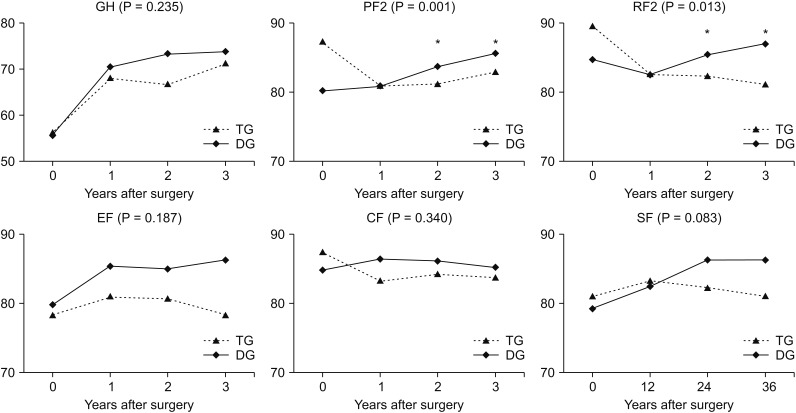
Fig. 3
Chronological changes in quality of life (symptom scales and items of EORTC QLQ-C30). EORTC, European Organisation for the Research and Treatment of Cancer; FA, fatigue; NV, nausea and vomiting; PA, pain; DY, dyspnea; SL, insomnia; AP, appetite loss; CO, constipation; DI, diarrhea; FI, financial difficulties; TG, total gastrectomy; DG, distal gastrectomy. *Significant changes between the groups and over time.

Fig. 4
Chronological changes in quality of life (EORTC QLQ-STO22). EORTC, European Organisation for the Research and Treatment of Cancer; XDG, dysphagia; Xpain, pain; XRflx, reflux symptoms; XEatR, eating restrictions; Anx, anxiety; DM, having a dry mouth; T, taste; BI, body image; HL, hair loss. *Significant changes between the groups and over time.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download