INTRODUCTION
Congenital diaphragmatic hernia (CDH) is a group of conditions characterized by developmental defects of the diaphragm with an incidence of 1/2500–1/4000. The developmental defects are usually associated with pulmonary hypoplasia, abnormal pulmonary vascular development, and vasoreactivity. Both abnormal lung development in embryonic stage and unproper treatment after birth could lead to morbidity after birth [
1]. Most survivors showed reduced forced vital capacity for several weeks after the repair surgery, and mild-to-moderate airway obstruction may still persist into adolescence and young adulthood [
23].
Several studies have examined lung mechanics, functional residual capacity, and forced expiratory flows over the tidal range of breathing in CDH infants [
245]. Advances in infant pulmonary function techniques have improved the assessment of flow rates in infants and toddlers by avoiding airway intubation. In our study, noninvasive tidal breathing pulmonary function testing was applied to evaluate respiratory function. The aim of this study was to investigate the risk factors affecting postoperative lung function and ameliorate perioperative therapy in CDH.
METHODS
Study design and ethics approval
A retrospective study for CDH was performed from November 2016 through September 2018. The registration number of this study is ChiCTR1800017627 on the Chinese clinical trial center (
http://www.chictr.org.cn). The study was performed with strict accordance with Helsinki Declaration. The research was reviewed by the Ethics Committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and the approval number is XHEC-D-2018-053.
Clinical data acquisition
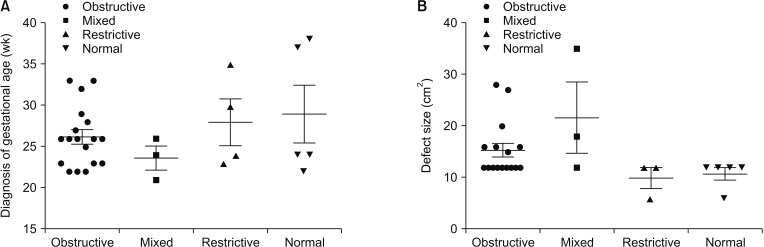
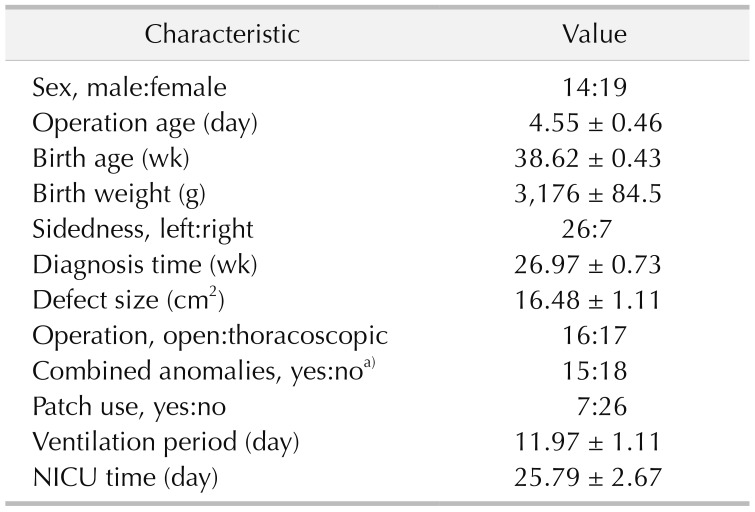
The medical records of all candidates were reviewed to obtain clinical data, including sex, prenatal diagnosis of gestational age (GA), birth weight, surgical approach, sidedness, defect size, ventilation period, days of neonatal intensive care unit and combined anomalies, including liver herniation, atrial septal defect, patent ductus arteriosus, and pulmonary arterial hypertension. All cases of CDH infants were diagnosed prenatally and underwent repair operation postnatally. Lung function was assessed by tidal volume analysis 3 weeks after surgery when most patients were in stable situation.
Tidal breathing pulmonary function testing
Pulmonary function testing was performed in all enrolled CDH infants within 3 weeks after operation. The method of tidal breathing was used to determine pulmonary function and assist in ruling out severe expiratory airway obstruction. Measurements were performed according to European Respiratory Society/American Thoracic Society guidelines [
67]. Informed consent was given by the children's legal guardians before testing. Flows and volumes were measured using a commercially available system (Master Screen Paed, JAEGER, Friedberg, Germany) connected via a low-dead-space face mask secured tightly. In this test, we used tidal breathing flow-volume analysis to determine the pulmonary function, through mask-to-monitor, by applying the flow sensor during quiet breathing, thus analyzing the all respiratory function changes. Measured parameters include: tidal volume by per kilogram (V
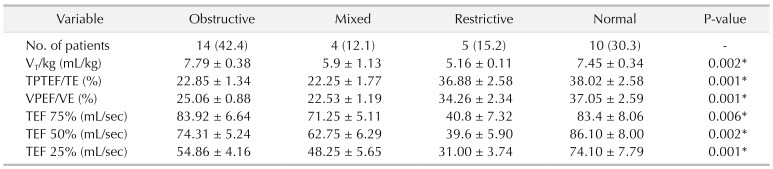
T/kg), time to peak tidal expiratory flow as a proportion of expiratory time (TPTEF/TE), volume to peak expiratory flow as a proportion of exhaled volume (VPEF/VE), expiratory flow when 75%, 50%, and 25% of tidal volume remains in the lungs (TEF 75%, TEF 50%, TEF 25%). The normal value of V
T/kg was 6–10 mL/kg. The normal value of TPTEF/TE and VPEF/VE was 28–55 mL/sec.
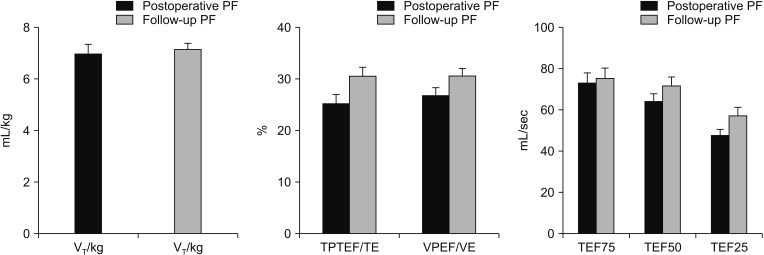
All infants were insured under quiet breathing before testing. The state of body temperature and pressure saturated conditions of the testing environment were 37℃ and 760 mmHg, respectively. Each lung function testing was repeated 3 times, and the average value was recorded as definitive result in the research. The possible correlations between clinical characteristics and postoperative lung function were analyzed to identify the risk factors correlated with postoperative lung function. All the infants with pulmonary dysfunction were treated with atomizing airway management to recover pulmonary function. The pulmonary function of the CDH patients was followed for 3 months after discharge from hospital.
Data analysis
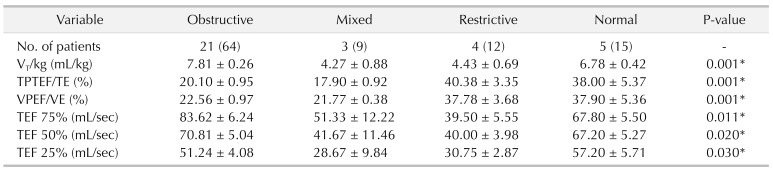
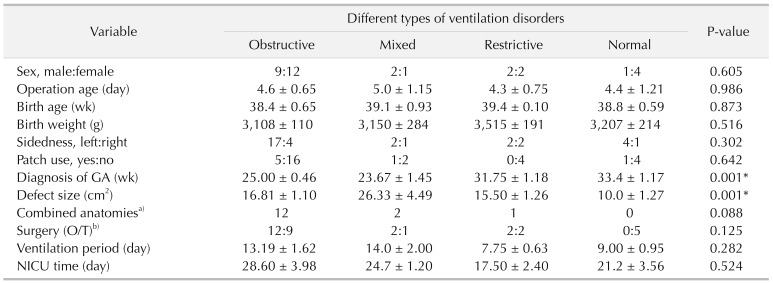
All patients were classified into 4 groups according to pulmonary function: obstructive ventilatory disorder group (group 1), mixed ventilatory disorder group (group 2), restrictive ventilatory disorder group (group 3), and normal lung function group (group 4). We used statistical R programming to analyze the possible correlation between operative characteristics and lung function in all 4 groups. Demographic variables were analyzed to detect potential risk factors for pulmonary function and severity of disease. Patient characteristics and pulmonary function parameters were presented as mean ± standard error of the mean. The data of all groups were analyzed by multivariate analysis of variance in IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). The group-wise changes in lung function and correlation with patient characteristics were determined using Pearson correlation. Statistical significance for all analysis was determined at the P < 0.05 level.
DISCUSSION
CDH is one of the most severe and life-threatening congenital diseases in the neonatal period characterized by unilateral or bilateral phrenic dysplasia, abdominal viscera herniation, and a major pathophysiological change. It usually combines a developmental defect of the diaphragm with pulmonary hypoplasia, abnormal pulmonary vascular development, and vasoreactivity. As a result, infants with CDH usually have variable degrees of pulmonary hypoplasia at birth. Functional and microstructural changes in lungs can persist into adulthood of most CDH patients [
3]. Perioperative relative factors may affect pulmonary function in CDH infants. However, few studies of lung function over the first years of CDH infants have been reported. Herein, we analyzed the correlation between operative characteristics and pulmonary function parameters and found that defect size, diagnosis time, and ventilation time may be associated with postoperative pulmonary function.
The most significant result of our work is to verify risk factors of pulmonary dysfunction in CDH infants after surgery consistent with previous reports [
235]. Generally, normal values of V
T/kg (tidal volume per kilogram) in children are 6–10 mL/kg, and decreased values indicate restrictive pulmonary disease. Obstructive disease, especially moderate or severe, tidal volume could decrease. TPTEF/TE is one of the main indicators reflecting airway obstruction. VPEF/VE is another parameter reflecting airway obstruction, and its changes are nearly consistent with the ratio of TPTEF/TE. The correlation between them reaches 90%. TPTEF/TE and VPEF/VE are the most sensitive indicators in neonatal respiratory function measurement. The heavier the obstruction is, the lower the ratio is. Normal values of TPTEF/TE and VPEF/VE in infants are 28%–55%. There is also a point of view that the expiratory flow velocity when exhaling different tidal volume, such as TEF 25%, TEF 50%, TEF 75%, is also a sensitive indicator for detection of small airway obstruction. Patients with obstructive ventilatory disorders generally showed lower TPTEF/TE and VPEF/VE values. Patients with restrictive ventilatory disorders showed lower V
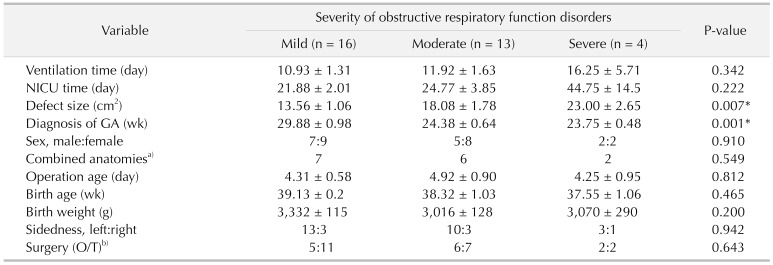
T/kg values. In our research, larger defect size was found in patients with mixed ventilatory disorders and obstructive ventilatory disorders. Diagnosis time was also detected much earlier in patients with severe obstructive ventilatory disorders. Recently, a long-term study of CDH repair also demonstrated that larger defect size was associated with higher rates of early recurrence in CDH infants [
8]. Other studies also demonstrated that prenatal diagnosis was correlated with poor outcome of CDH infants [
910].
The degree of pulmonary function impairment was also correlated both with markers of the initial degree of pulmonary hypoplasia and the duration of mechanical ventilation [
2]. Meanwhile, children with CDH have primary disease with severe lung dysplasia, and such patients require ventilator-assisted respiration after birth, which may significantly aggravate lung injury of children and cause severe tissue damage. However, there is no significant difference between ventilation time and ventilatory disorders in our research.
The mechanisms of pulmonary hypoplasia in CDH infants have been controversial. It has been suggested that an early embryogenic alteration of the pulmonary vasculature and parenchymal (and diaphragmatic) development, as well as external compression from herniated intra-abdominal contents, stifle pulmonary vasculature/parenchymal development [
11]; Guilbert et al. [
12] showed that pulmonary hypoplasia and the intercept in lung development occur before the normal closure of the diaphragm and they are partially independent from the mechanical compression of the ascended abdominal organs. These procedures are amalgamated into the dual-hit hypothesis. The first hit, possibly genetic or environmental, probably occurs at an early phase before the formation of the diaphragm, which would arrest pulmonary development in both lungs. Lung compression by the abdominal organs at a later phase (second hit) would then worsen the ipsilateral hypoplasia, explaining why both lungs are usually hypoplastic, though the ipsilateral lung is further influenced than the contralateral [
11]. Meanwhile, human postmortem studies confirmed that CDH patients had severe dysplasia in the ipsilateral lung, and only mild dysplasia of the contralateral lung [
13]. Researches in newborns with CDH had shown that CDH was composed of fewer alveoli and reduced gas-exchange surface area, significantly thickened alveolar walls, arteries of a smaller diameter than normal, larger alveoli, and increased interstitial tissue in alveolar development [
12]. It indicated that pulmonary hypoplasia and dysfunction existed in CDH patients, and these patients might be vulnerable to poor pulmonary function outcome.
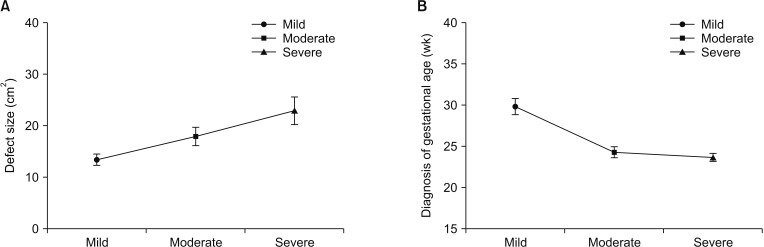
Our pulmonary function test results confirmed that different types of ventilation disorder existed in CDH patients after surgery. Children with mild diaphragmatic hernia may be prone to restrictive ventilatory disorder postoperatively; while moderate or severe diaphragmatic hernia may be prone to obstructive lung ventilation or mixed disorders. Meanwhile, long-term follow-up study has identified pulmonary function disorder existed in most patients with CDH after surgery [
31415]. Earlier diagnosis time was also associated with more severe ventilatory disorder. It is important to note that antenatal care is of great significance for observing some severe diseases. Therefore, postoperative pulmonary function rehabilitation is of great significance in improving pulmonary function abnormality after diaphragmatic hernia.
Our study preliminarily analyzed risk factors associated with postoperative outcomes of pulmonary function in infants with CDH. Our results showed that defect size, ventilation time, and prenatal diagnosis of GA were risk factors correlating with postoperative pulmonary function of CDH infants. To our knowledge, this is the first report of risk factors affecting postoperative pulmonary function in CDH infants. Long-term follow-up study should further explore the pulmonary function modification in CDH patients at several years after surgery, and whether it might enhance medicine intervention in pulmonary rehabilitation. One limitation of our work is insufficient number of cases; however, the small number of infants with CDH managed in our center further highlights the attention needed for the rehabilitation of CDH patients' respiratory function after operation. Also, a collaborative multidisciplinary research ought to be established to facilitate pulmonary function rehabilitation and improve clinical outcomes of CDH patients.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download