Abstract
Purpose
Pancreatic duct decompression relieves pancreatic duct stone (PDS)-associated abdominal pain, though a consensus indication for the drainage procedure of the main pancreatic duct (MPD) is lacking. Moreover, major prognostic factors for postsurgical long-term pain relief and recurrence are largely unknown.
Methods
The clinical outcomes of 65 consecutive PDS patients undergoing surgery from 2008–2012 with 3+ years of follow-up were assessed.
Results
At postsurgical follow-up (median, 4.5 years; range, 3–7 years; procedure: Partington, n = 32; Frey, n = 27; pancreatoduodenectomy, n = 3; distal pancreatectomy, n = 3), the early complication and complete stone clearance rates were 29.2% and 97%, respectively. Long-term, complete and partial pain relief were 93.9%, 83.1%, and 10.8%, respectively. The risk of pancreatic fistula was higher in the <8 mm group than in the >8 mm group (P < 0.05), and 80% of the pancreatic fistula cases occurred in the <8 mm group. A shorter pain duration (P = 0.007), smaller MPD diameter (P = 0.04), and lower Izbicki pain score (P < 0.001) predicted long-term pain relief. Pain recurrence after initial remission occurred in 5 patients and was only related to pain duration (P = 0.02). Stone recurrence and pancreatic exocrine functional and endocrine functional deterioration occurred in 2, 5, and 11 patients, respectively.
Conclusion
Surgery provides excellent stone clearance, long-term pain relief, and acceptable postoperative morbidity. Using 8 mm as the criterion for drainage surgery can minimize the postoperative pancreatic fistula risk. Individualized and timely surgical treatment may improve the effect of surgery.
Due to predisposing factors, such as long-term alcoholism, the morbidity of chronic pancreatitis (CP) has increased in the last few decades. The incidence of CP is between 2 and 200 per 100,000 people globally [1] and 13 per 100,000 people in China [2]. Inflammation associated with CP induces stricture formation in the main pancreatic duct (MPD) followed by the formation of pancreatic duct stones (PDSs). PDSs present as the most common and typical histological change in CP, and they lead to stasis of the pancreatic juice, high pressure in the pancreatic duct, and progressive inflammation of CP. CP is characterized by permanent damage to the pancreatic structure and function, ultimately resulting in intractable abdominal pain with progressive exocrine and endocrine insufficiency [3].
Pain is usually the predominant complaint of patients with CP, and intractable pain that is resistant to drug treatment is the main surgical indication for CP with PDSs [4]. Although the etiology of pain in PDSs remains unclear, perineural inflammation, and increased intraductal pressure caused by a dilated pancreatic duct, which is secondary to obstruction, are deemed responsible for the induction of pain [5]. Therefore, surgical approaches for pain in which medical treatment is ineffective are based on differing pathophysiological theories of the etiology of pain, including resection (pancreaticojejunostomy), and drainage/decompression surgery (such as the Partington and Frey procedures) [5]. Although extracorporeal shock wave lithotripsy (ESWL) or endoscopy is recommended as the first-line treatment for pancreatic stones by the European Society of Gastrointestinal Endoscopy (ESGE) [6] and Japanese Study Group for Pancreato-Biliary Lithiasis, surgery in the treatment of painful CP with PDSs is valuable and necessary in economically disadvantaged areas and developing countries, including China. The reasons lie not only in the corresponding special medical equipment and skilled endoscopic physicians that are needed for ESWL and endoscopy, but also in the fact that cases of multiple pancreatic duct strictures are not appropriate for ESWL or endoscopy [5]. To resolve multiple pancreatic duct strictures and the increased pancreatic duct pressure they cause, a drainage operation (the Partington procedure) and extended drainage operation (such as the Frey procedure) are usually chosen [5]. Most scholars and relevant organizations, including the Japanese Society of Gastroenterology, believe that pancreaticojejunostomy should be performed when the MPD is not dilated and that drainage or extended drainage surgery should be selected when the MPD is dilated [7]. However, there is no uniform standard for the specific criteria for pancreatic duct dilation or the diameter of the MPD. Therefore, this study aimed to describe the short- and long-term outcomes of surgery for CP with PDSs in China and mainly focused on the specific criteria for the suitability of the MPD for drainage or extended drainage surgery.
From January 2008 to November 2012, we collected data from 65 consecutive patients out of 81 patients with PDSs undergoing surgery at the People's Hospital of Zhengzhou University and analyzed the data. Patients who had not continued long-term follow-up in our hospital were contacted by telephone, and data from routine clinical evaluations and laboratory testing were collected. Patients whose data were lost during the follow-up period or who were diagnosed with pancreatic cancer by postoperative pathology were excluded from the study.
This study is cleared with the Institution Review Board (IRB) of Henan Provincial People's Hospital and the Second Affiliated Hospital of Chongqing Medical University and the associated IRB number is No. 8180102469. We have received written informed consent for publication from the patients.
Surgery was performed by experienced hepatobiliary surgeons in our hospital. Intraoperative ultrasound was used to estimate the locations of the PDSs and whether the calculi were completely removed. When the MPD was dilated more than 6 mm without inflammatory masses at the pancreatic head, pancreatolithotomy plus side-to-side pancreaticojejunostomy (Partington operation) was performed. When the dilation was more than 6 mm with inflammatory masses, the Frey procedure was selected. Regarding inflammatory masses, given that the pancreatic head is the pacemaker of the chronic inflammatory lesion, we also chose the Frey procedure when there were no inflammatory masses at the pancreatic head. When a malignant tumor of the pancreatic head was suspected by preoperative examination or intraoperative frozen section analysis, pancreatoduodenectomy (PD) was applied. When PDSs were located in the left pancreatic duct and the diameter of the MPD was smaller than 5 mm, distal pancreatectomy (DP) was performed.
The clinical data of the 65 patients from their hospital stay were analyzed, including age, sex, body mass index, pain duration, symptoms, aetiologies, laboratory findings, and imaging examination results. Data on the stone characteristics and MPD diameter were intentionally obtained.
The primary outcome measure was pain relief at the end of follow-up. The secondary measures included morbidity, mortality, complete stone clearance, stone recurrence, length of hospital stay, and changes in exocrine and endocrine pancreatic function. Pain relief was classified as complete (Izbicki pain score, ≤10) or partial (Izbicki pain score, >10 after a decrease of >50%) [8]. Stone removal was defined as complete if there was no positive result in the MPD on imaging examination. Endocrine insufficiency was defined as a required treatment for glycaemic control. Patients were considered to have exocrine insufficiency if the elastase level was less than 200 µg per gram of feces.
Continuous variables with normal distribution are expressed as the mean ± standard deviation. Categorical variables are presented as frequencies (%). The statistical analyses of enumeration data were evaluated using the chi-square test or Fisher exact test. Predictive factors of long-term pain recurrence after surgery were determined by multivariable logistic regression analysis. All statistical analyses were performed using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). A P-value of less than 0.05 was considered significant.
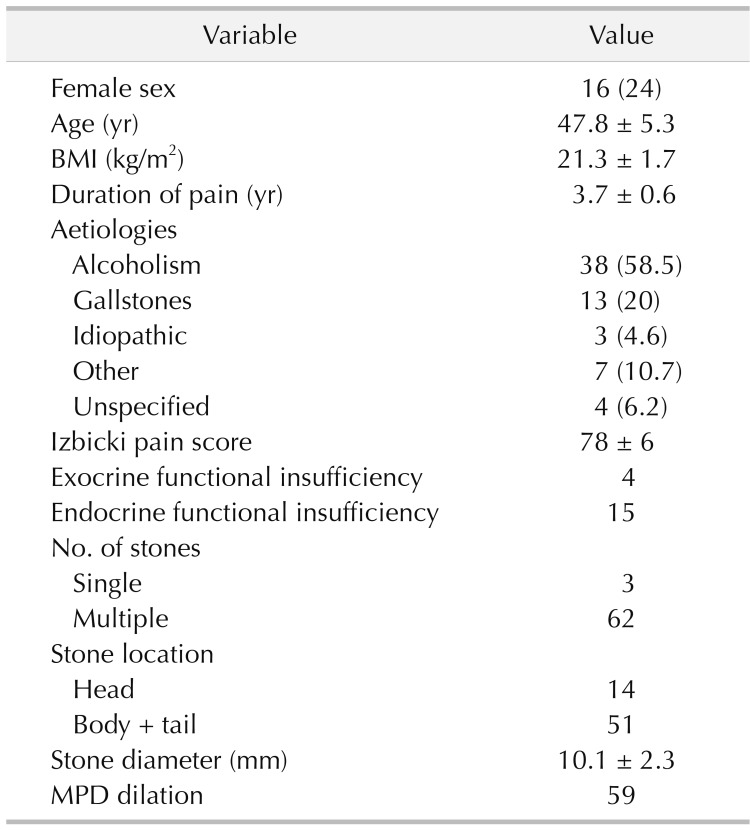
Of the 81 patients with PDSs who underwent surgery in Henan Provincial People's Hospital and the Second Affiliated Hospital of Chongqing Medical University between 2008 and 2012, 12 patients were lost to follow-up, and 4 patients with pancreatic cancer were excluded. Therefore, data on 65 patients with a median follow-up time of 4.5 years (range, 3–7 years), including 49 men and 16 women with an average age of 47.8 ± 5.8 years, were included in the study. The baseline characteristics of these patients are shown in Table 1. The main aetiologies included long-term alcoholism (58.5%), gallstones (20%), and idiopathic pancreatitis (4.6%). All of the patients with PDSs suffered from abdominal or back pain and were routinely evaluated by ultrasound. Forty-five patients were evaluated by magnetic resonance cholangiopancreatography (Fig. 1A), and 33 were evaluated by contrast-enhanced CT (Fig. 1B). Most patients (95.3%) had multiple stones with dilations (90.7%) and strictures (87.7%) of the MPD.
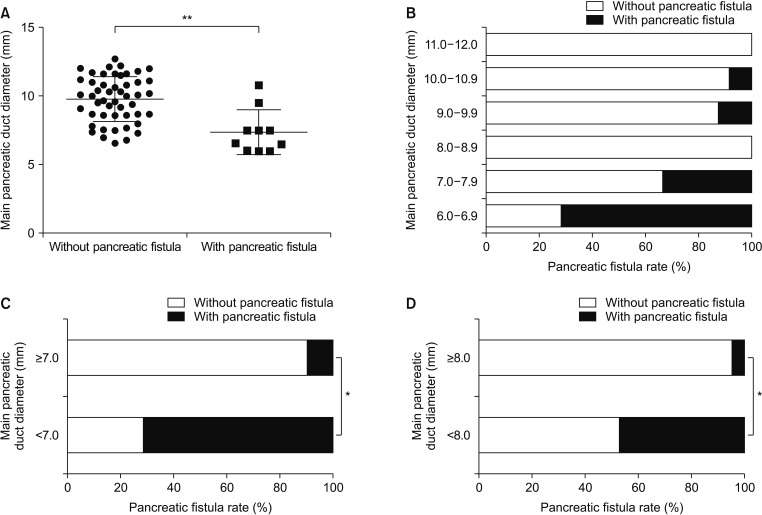
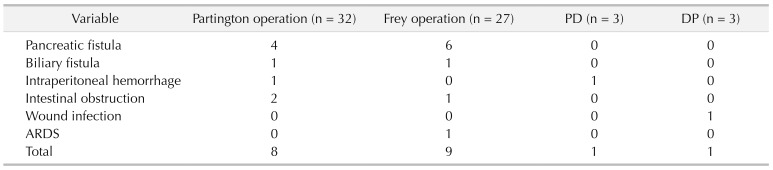
The Partington operation was performed in 32 patients (Fig. 2), the Frey procedure was performed in 27 patients (Fig. 3), PD was performed in 3 patients, and DP was performed in 3 patients (Table 2). Of the 65 patients, 19 experienced early complications, including pancreatic fistula (15.4%), biliary fistula (3.1%), intraperitoneal haemorrhage (3.1%), intestinal obstruction (4.6%), wound infection (1.5%), and ARDS (1.5%). One patient died after PD due to severe pancreatic fistula and following intraperitoneal hemorrhage. It is known that the outcome of MPD diameter (pancreatic duct) expansion in decompressive surgery mainly involves the incidence of postoperative complications. Considering the high incidence rate of pancreatic fistula and its strong connection with pancreatic duct expansion, we evaluated the correlations among the incidence rate of pancreatic fistula and the MPD diameter in cases that had decompressive surgery (Partington and Frey operations). The results showed that the MPD diameter of the pancreatic fistula group was significantly lower than that of the nonpancreatic fistula group (Fig. 4). Among the groups with different diameters, the incidence of pancreatic fistula was the highest in the 6.0- to 6.9-mm diameter group, which was significantly higher than that in the other groups. In addition, the risk of pancreatic fistula was much higher in the <8 mm group than in the >8 mm group (Fig. 4). Moreover, more than 90% of the pancreatic fistula cases occurred in the <8 mm case group (Fig. 4).
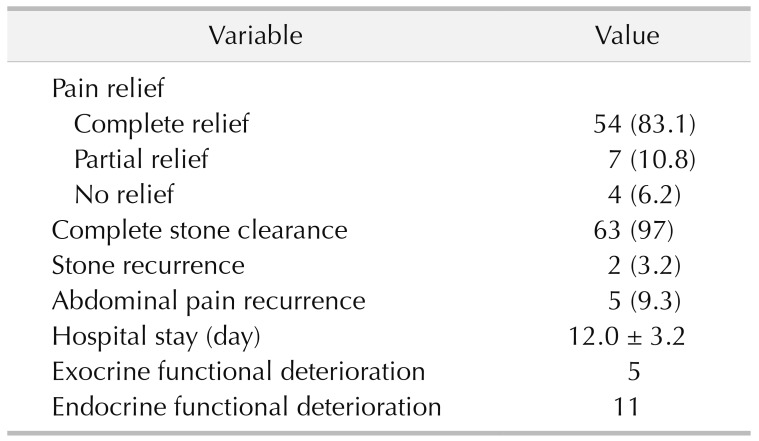
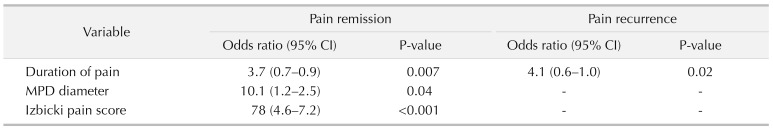
The outcomes are summarized in Table 3. Complete stone clearance occurred in 63 patients. Residual pancreatolithiasis, located in the branch pancreatic duct and accessory pancreatic duct, occurred in 2 patients. At the end of follow-up, the complete and partial pain relief rates were 83.1% and 10.8%, respectively. Pain recurrence after initial remission occurred in 5 patients, stone recurrence occurred in 2 patients, deterioration of pancreatic exocrine function occurred in 5 patients, and deterioration of endocrine function occurred in 11 patients. According to the multivariate regression analysis (Table 4), a shorter duration of pain (P = 0.007), a smaller diameter of the dilated MPD (P = 0.04), and a lower Izbicki pain score (P < 0.001) predicted long-term pain relief. Pain recurrence was only related to pain duration (P = 0.02) (Table 4).
As described in the introduction, consensus regarding the criteria for the drainage procedure of CP with PDSs has not been established. Additionally, data on the major prognostic factors of long-term pain relief and recurrence rates after surgery are still lacking. Our findings revealed that surgery for pancreatolithiasis had satisfactory long-term outcomes with acceptable short-term postoperative morbidity. Additionally, a shorter pain duration, smaller MPD diameter, and lower Izbicki pain score were associated with better long-term pain relief; pain recurrence was only related to pain duration. Moreover, using 8 mm as the standard can maximize the avoidance of postoperative pancreatic fistula risk.
The principle purpose of surgical intervention in CP is to relieve pain and preserve as much of the pancreatic parenchyma as possible. Different surgical procedures can be chosen according to the location of the stones in the pancreatic duct, the dilation, and stenosis of the MPD, whether or not a mass or pseudocyst is present. The Partington and Rochelle procedure is common in surgical practice, with low morbidity and mortality. However, approximately 20% of patients with CP have a dominant inflammatory mass within the pancreatic head, which is identified as the leading site of the disease and causes the pain sensations of some patients [9]. The Frey procedure has been recommended by many surgical scholars because it allows for better pain relief than the Partington and Rochelle procedure [910]. Therefore, we also chose the Frey procedure, even without inflammatory masses at the pancreatic head, and there were no differences between the 2 procedures in terms of the time of operation, volume of lost tissue, or rate of early complications. The Frey and Beger procedures are reasonable options for patients with inflammatory masses at the pancreatic head and are equally effective in terms of providing pain relief [1112], while the Beger procedure can be performed to remove a potential tumor within the pancreatic head [13]. We decided on the operation type according to the results of the intraoperative frozen section analysis. When a malignant tumor of the pancreatic head was suspected on preoperative examination or intraoperative frozen section analysis, PD, but not the Beger procedure, was applied to generate a greater scope of surgical resection and improve the curative effect [14]. Very few studies have discussed the optimal timing of surgical intervention. We found that a shorter duration of pain, a smaller diameter of the dilated MPD, and a lower Izbicki pain score predicted long-term pain relief. We consider that the abovementioned factors hint that the shorter the onset time is, the milder the pancreatic lesions and peripheral nerve pain infringement will be. That is, timely surgical intervention seemed to be more beneficial in the advance phase of CP.
Surgery can be performed to remove stones as much as possible and relieve MPD obstruction to achieve better decompression [15], thus reducing intrapancreatic pressure either within the pancreatic duct or in the pancreatic parenchyma [16]. It has long been recognized that intrapancreatic neural damage and alterations correlate with abdominal pain, which is difficult to relieve by simple drainage [17]. This can explain why some patients only achieved partial pain relief even without pain relief after surgery in our study. As a less invasive therapy, ESWL is a very successful modality that has historically been used in the treatment of renal stones and gallstones. However, ESWL alone is not satisfactory, with a low rate of complete stone clearance and a high rate of stones and abdominal pain recurrence in the treatment of PDSs. In a Japanese multicentre study, which was performed on 916 patients managed in 34 institutions, complete stone clearance was achieved in 49.4% of the patients after ESWL alone [18]. Similarly, as described in the ESGE clinical guidelines, the complete stone clearance rate reached only 60% after endoscopy alone [8]. PDSs located in the branch duct with diameters >10 mm or >3 are not suitable for endoscopy [19]. With the ability to fragment large stones, stone fragmentation achieves 89% to 92.0% by ESWL. Therefore, ESWL and subsequent endoscopy are used to significantly increase the rate of complete stone clearance, which is achieved in 41% to 90% (mean 60%) of cases [820]. Even so, surgery provides more thorough stone clearance than ESWL with endoscopy [61821]. In our study, the complete stone clearance rate was 97%. PDSs were partially removed in 2 patients because the stones impacted the accessory pancreatic duct and branch. In a previous study, early complications after ESWL and endoscopy were rarer than after surgery [91820]. In our study, early complications after surgery were noted in 9 patients. An individualized surgical treatment based on the location and different pathological types of CP with PDSs could lead to acceptable short-term postoperative complications and excellent long-term outcomes.
Once surgery is deemed necessary, the choice of surgical procedure should be made. The available surgical procedures are resection, drainage, and extended drainage surgery. Among the 3 types of surgical procedures, the main advantage of the resection procedure is complete resection of the stenosis of the pancreatic duct and the prevention risk of oncogenesis [5]. Drainage procedure, on the other hand, is deemed to be advantageous in terms of short-term complications because it is a simple surgical procedure [5]. Extended drainage operation has both of the above advantages [5]. Although the 3 surgical methods have their own characteristics, they are basically consistent in terms of their pain relief rates and long-term prognoses. The choice of method is mainly based on the pathological morphology of the patient's pancreas. Most scholars believe that when there is a pancreatic inflammatory mass, resection should be selected and that when the MPD is dilated, drainage should be selected [22]. And, when both findings are present, the extended drainage operation should be selected [22]. However, there is no uniform standard for the specific definition of “main pancreatic duct dilation”. Six millimeters, 7 mm and 10 mm are recommended by different scholars as the MPD diameter standard for drainage surgery. Undoubtedly, drainage of the MPD to expand the pancreas reflects the value of reducing pancreatic duct pressure to relieve pain due to PDSs. At the same time, most scholars believe that although there are many factors affecting pancreatic fistula, including the preoperative nutritional status, pancreatic texture, and surgical experience of the surgeon, the MPD diameter is undoubtedly one of the most important factors [22]. The more obvious the degree of pancreatic duct dilatation is, the lower the risk of postoperative complications, especially pancreatic fistula, will be, which is an important reason for drainage or combined surgery requiring pancreatic duct expansion [2223]. The results of this study showed that the incidence of pancreatic fistula was significantly decreased when the diameter of the pancreatic duct was greater than 7 mm or 8 mm, suggesting that 7 mm and 8 mm were the standard. In addition, in this study, 90% of pancreatic fistulas occurred in patients with pancreatic duct diameters below 8 mm, suggesting that taking 8 mm as the standard can minimize the risk of postoperative pancreatic fistula.
In conclusion, the limitations of this study were its retrospective design, small sample size, and single-center experience. A further largescale prospective randomized study is needed to further estimate the efficacy of surgery for patients with PDSs. Based on its long-term results, our study has shown that surgery provides excellent stone clearance and long-term pain relief with acceptable postoperative morbidity. An individualized and timely surgical treatment regimen may improve the effect of surgery. The degree of MPD dilatation is closely related to postoperative complications, especially the incidence of pancreatic fistula. A pancreatic duct diameter of 7 mm is an effective standard for reducing the risk of postoperative pancreatic fistula, while a diameter of 8 mm as the standard can maximize the avoidance of postoperative pancreatic fistula risk.
Notes
References
1. Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol. 2011; 106:2192–2199. PMID: 21946280.

2. Garg PK, Tandon RK. Survey on chronic pancreatitis in the Asia-Pacific region. J Gastroenterol Hepatol. 2004; 19:998–1004. PMID: 15304116.

3. Ketwaroo GA, Freedman SD, Sheth SG. Approach to patients with suspected chronic pancreatitis: a comprehensive review. Pancreas. 2015; 44:173–180. PMID: 25675419.
4. Talukdar R, Murthy HV, Reddy DN. Role of methionine containing antioxidant combination in the management of pain in chronic pancreatitis: a systematic review and meta-analysis. Pancreatology. 2015; 15:136–144. PMID: 25648074.

5. Isaji S. Has the Partington procedure for chronic pancreatitis become a thing of the past? A review of the evidence. J Hepatobiliary Pancreat Sci. 2010; 17:763–769. PMID: 19779664.

6. Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, Arvanitaki M, et al. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012; 44:784–800. PMID: 22752888.

7. Inui K, Masamune A, Igarashi Y, Ohara H, Tazuma S, Sugiyama M, et al. Management of pancreatolithiasis: a nationwide survey in Japan. Pancreas. 2018; 47:708–714. PMID: 29851750.
8. Choi EK, McHenry L, Watkins JL, Sherman S, Fogel EL, Cote GA, et al. Use of intravenous secretin during extracorporeal shock wave lithotripsy to facilitate endoscopic clearance of pancreatic duct stones. Pancreatology. 2012; 12:272–275. PMID: 22687384.

9. Zheng Z, Xiang G, Tan C, Zhang H, Liu B, Gong J, et al. Pancreaticoduodenectomy versus duodenum-preserving pancreatic head resection for the treatment of chronic pancreatitis. Pancreas. 2012; 41:147–152. PMID: 21775913.

10. Bachmann K, Tomkoetter L, Erbes J, Hofmann B, Reeh M, Perez D, et al. Beger and Frey procedures for treatment of chronic pancreatitis: comparison of outcomes at 16-year follow-up. J Am Coll Surg. 2014; 219:208–216. PMID: 24880955.

11. D'Haese JG, Ceyhan GO, Demir IE, Tieftrunk E, Friess H. Treatment options in painful chronic pancreatitis: a systematic review. HPB (Oxford). 2014; 16:512–521. PMID: 24033614.
12. Ceyhan GO, Demir IE, Maak M, Friess H. Fate of nerves in chronic pancreatitis: neural remodeling and pancreatic neuropathy. Best Pract Res Clin Gastroenterol. 2010; 24:311–322. PMID: 20510831.

13. Keck T, Wellner UF, Riediger H, Adam U, Sick O, Hopt UT, et al. Long-term outcome after 92 duodenum-preserving pancreatic head resections for chronic pancreatitis: comparison of Beger and Frey procedures. J Gastrointest Surg. 2010; 14:549–556. PMID: 20033344.

14. Bachmann K, Tomkoetter L, Kutup A, Erbes J, Vashist Y, Mann O, et al. Is the Whipple procedure harmful for long-term outcome in treatment of chronic pancreatitis? 15-years follow-up comparing the outcome after pylorus-preserving pancreatoduodenectomy and Frey procedure in chronic pancreatitis. Ann Surg. 2013; 258:815–820. discussion 820-1. PMID: 24096767.

15. Ahmed Ali U, Issa Y, Bruno MJ, van Goor H, van Santvoort H, Busch OR, et al. Early surgery versus optimal current step-up practice for chronic pancreatitis (ESCAPE): design and rationale of a randomized trial. BMC Gastroenterol. 2013; 13:49. PMID: 23506415.

16. Del Chiaro M, Segersvard R, Lohr M, Verbeke C. Early detection and prevention of pancreat ic cancer: is it real ly possible today? World J Gastroenterol. 2014; 20:12118–12131. PMID: 25232247.
17. Wilson GC, Sutton JM, Abbott DE, Smith MT, Lowy AM, Matthews JB, et al. Long-term outcomes after total pancreatectomy and islet cell autotransplantation: is it a durable operation? Ann Surg. 2014; 260:659–665. discussion 665-7. PMID: 25203883.
18. Suzuki Y, Sugiyama M, Inui K, Igarashi Y, Ohara H, Tazuma S, et al. Management for pancreatolithiasis: a Japanese multicenter study. Pancreas. 2013; 42:584–588. PMID: 23558239.
19. Nguyen-Tang T, Dumonceau JM. Endoscopic t reatment in chronic pancreatitis, timing, duration and type of intervention. Best Pract Res Clin Gastroenterol. 2010; 24:281–298. PMID: 20510829.
20. Tandan M, Reddy DN, Santosh D, Vinod K, Ramchandani M, Rajesh G, et al. Extracorporeal shock wave lithotripsy and endotherapy for pancreatic calculi-a large single center experience. Indian J Gastroenterol. 2010; 29:143–148. PMID: 20717860.

21. Milovic V, Wehrmann T, Dietrich CF, Bailey AA, Caspary WF, Braden B. Extracorporeal shock wave lithotripsy with a transportable mini-lithotripter and subsequent endoscopic treatment improves clinical outcome in obstructive calcific chronic pancreatitis. Gastrointest Endosc. 2011; 74:1294–1299. PMID: 21981815.

22. Tandan M, Talukdar R, Reddy DN. Management of pancreatic calculi: an update. Gut Liver. 2016; 10:873–880. PMID: 27784844.

23. Panek-Jeziorna M, Wierzbicki J, Annabhani A, Paradowski L, Mulak A. Pancreatic duct stones: a report on 16 cases. Adv Clin Exp Med. 2017; 26:609–613. PMID: 28691427.
Fig. 1
Imaging tests of pancreatic duct stones evaluated by magnetic resonance cholangiopancreatography (A) and computed tomography (B). (A) Multiple stones are detected in the body and tail of the pancreas. (B) The main pancreatic duct is severely dilated; multiple stones are detected in the body and tail of the pancreas.
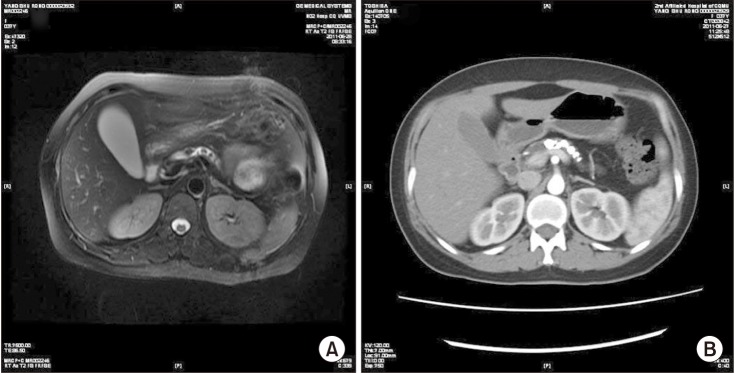
Fig. 2
Schematic diagram of the Partington surgery. (A, B) After longitudinal dissection of the pancreatic duct, scattered white calcified stones and pancreatic tissue fibrosis in the pancreatic duct were found. (C, D) The lateral anastomosis of pancreatic duct and jejunum. (E) Pancreatic duct stones removed from the pancreatic duct.
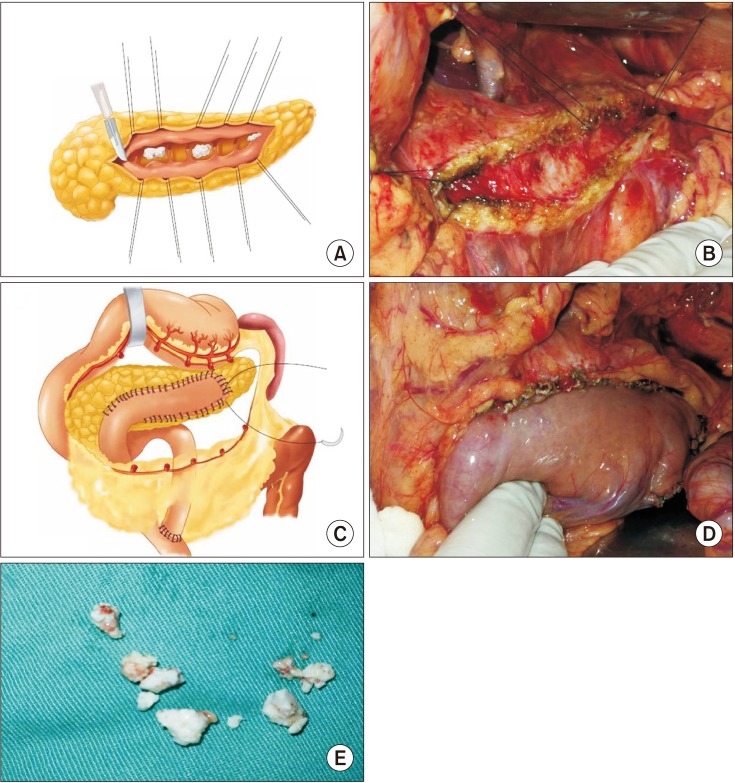
Fig. 3
Schematic diagram of Frey surgery. (A, B) The head of the pancreas was open and the stones were removed for decompression. (C, D) The anastomosis of pancreatic duct and jejunum. (E) Pancreatic duct stones removed from the pancreatic duct.
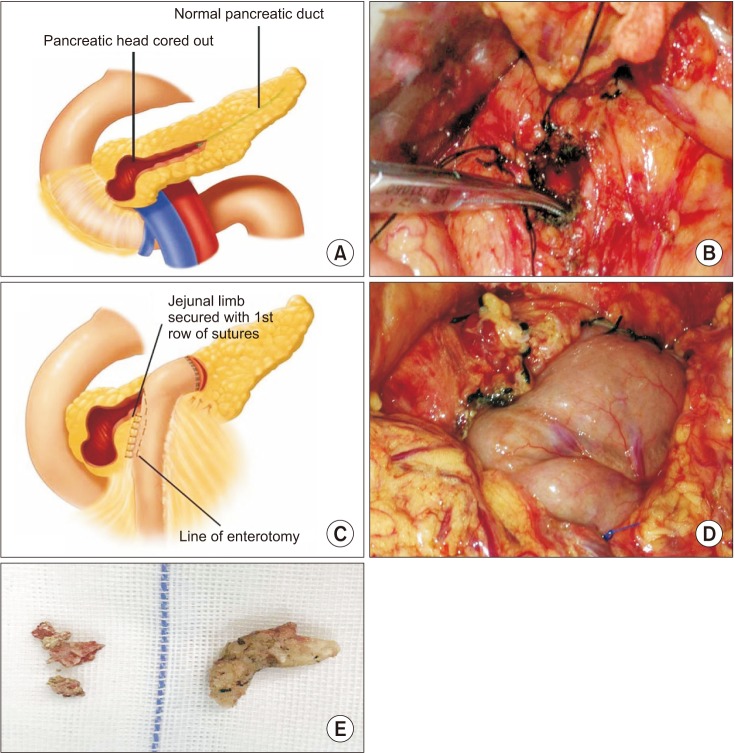
Fig. 4
Correlation between the main pancreatic duct diameter and pancreatic fistula incidence after the Partington or Frey operation. (A) The main pancreatic duct diameter in the group with (n = 10) or without pancreatic fistula (n = 49). (B) The incidence rate of pancreatic fistula in groups with different main pancreatic duct diameters. (C, D) The incidence rate of pancreatic fistula in groups according to different main pancreatic duct diameter criteria (7 mm or 8 mm) analyzed using the chi-square test. *P < 0.05. **P < 0.01.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download